SG EN ESTUDIO PLANET Y PRIME LLD
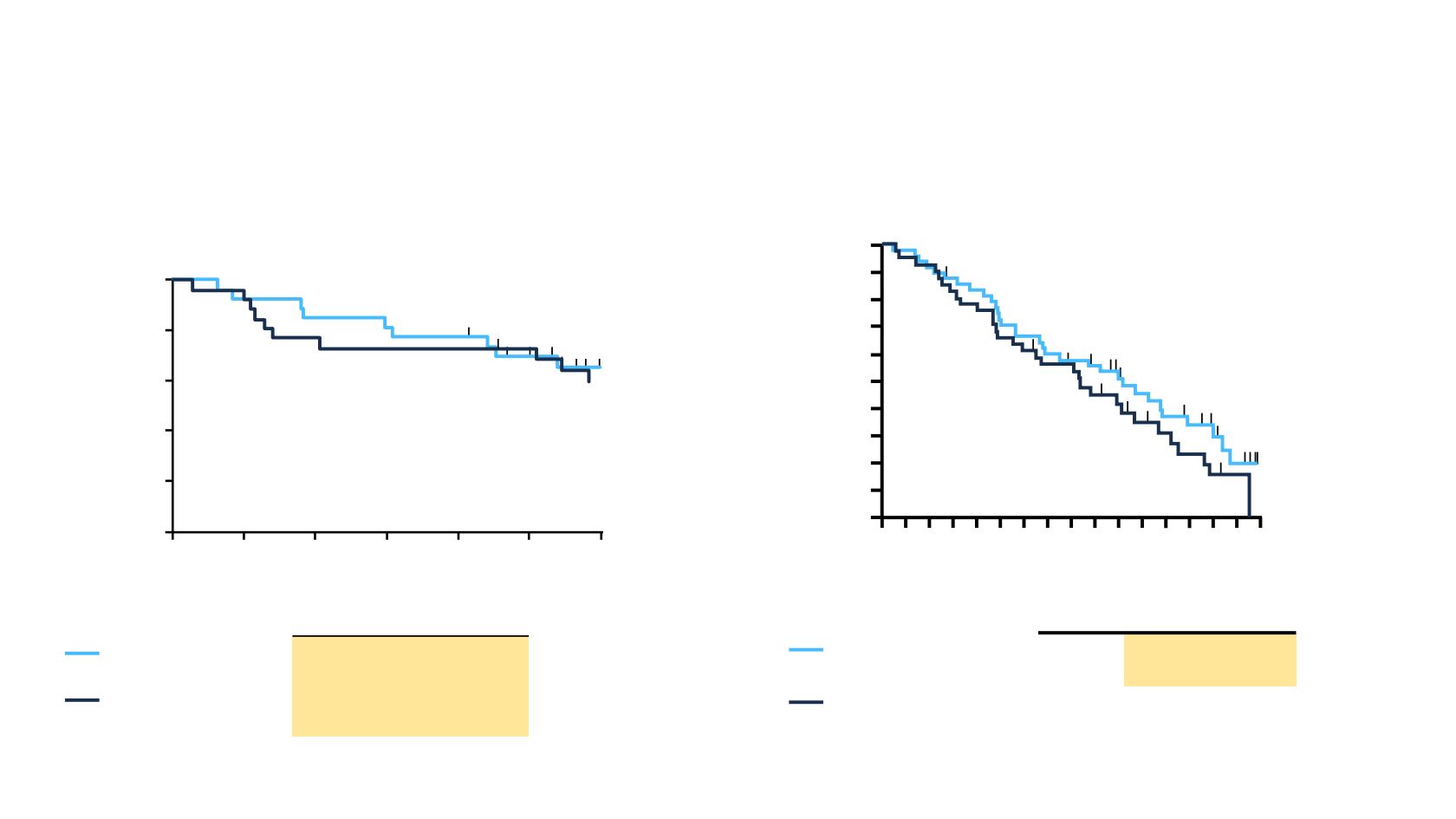
RAS WT patients
Median, months (95% CI)
Panitumumab
+ FOLFOX4 (n = 27)
39 (27–51)
Panitumumab
+ FOLFIRI (n = 26)
49 (31–56)
Carrato A, et al. Eur J Cancer 2017;81:191–202.
Peeters M, et al. EJC 2013; 49 (suppl 4):abstract MC13-0022 (and poster).
†
Panitumumab + FOLFIRI vs panitumumab + FOLFOX4; NE, not estimable.
HR
†
= 0.9 (95% CI, 0.5–1.9)
P = 0.824
PLANET
Months
Proportion alive (%)
6
0
100
80
60
40
20
0
12
18
24 30
36
Events
n (%)
Median (95% CI)
months
Panitumumab +
FOLFOX4 (n = 48)
32 (67)
40.7
FOLFOX4 (n = 41)
31 (76)
33.4
PRIME
0
20
40
60
80
100
90
70
50
30
10
Kaplan-Meier estimate
Months
0
64
4 8 12 16 20 24 28 32
60 56 52 48 44 40 36
HR = 0.71 (95% CI, 0.43–1.16)
P = 0.1737








