PALOMA-2. Tiempo hasta segundo tratamiento sistémico subsiguiente.
eters:
ulation, 80 patients
lap, 60 patients
ure 4)
.
clib + Letrozole,
EFS=40.4 months
I: 34.7–47.3
+ Letrozole,
EFS=29.9 months
I: 25.6–35.1
CDK 4/6 inhibitors
1 (0.7)
12 (12.4)
mTOR=mechanistic target of rapamycin.
*Percentages are calculated using n as a denominator
●
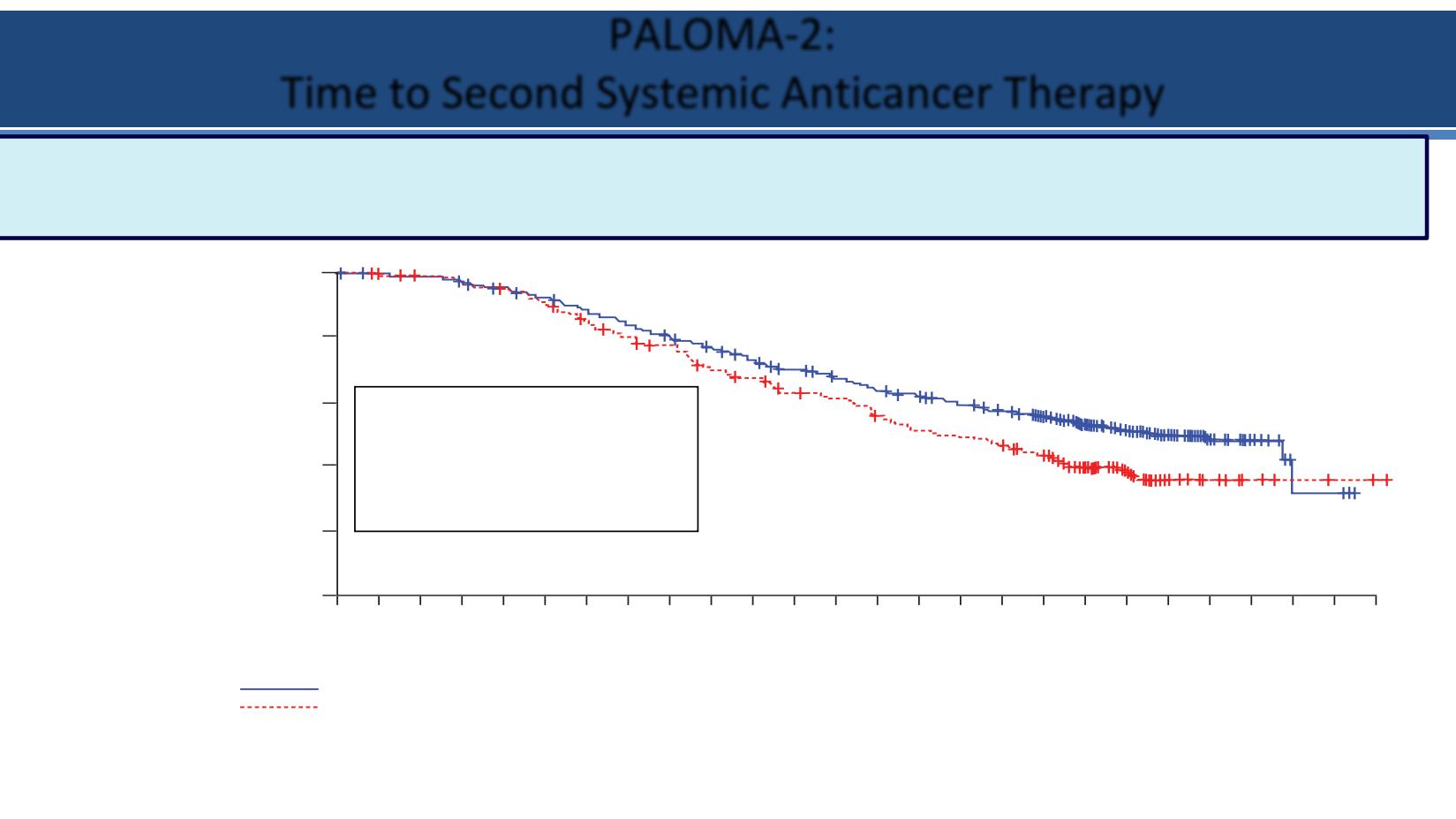
The 10-month difference of PFS benefit from palbociclib, observed in the primary PFS analysis, was
preserved, suggesting that the treatment benefit of the subsequent therapies was not compromised by
palbociclib plus letrozole
(Figure 5)
.
Figure 5.
Time to Second Subsequent Systemic Anticancer Therapy
0
Time, months
0
20
40
60
80
100
Patients at risk, n:
444
222
2
439
219
4
437
215
6
427
210
8
414
206
10
400
194
12
381
180
14
362
167
16
344
160
18
327
144
20
308
137
22
291
125
24
275
121
26
263
109
28
250
100
30
239
96
32
224
90
34
206
81
36
169
58
38
103
36
40
57
17
42
29
9
44
14
5
46
4
3
48
3
2
50
0
1
Hazard Ratio=0.72,
(95% CI, 0.58–0.90), 1-sided
P
<0.005
Palbociclib + Letrozole,
Median EFS=38.8 months
95% CI: 34.4–NE
Placebo + Letrozole,
Median EFS=28.8 months
95% CI: 25.7–33.5
Event-free Survival, %
EFS=event-free survival; NE=not estimable.
Rugo et al. Presentado SABCS 2017. P5-21-03
Time to Second Subsequent Systemic – Anticancer Therapy
Time to Second Systemic Anticancer Therapy
The 10-month difference of PFS benefit from palbociclib, observed in the primary PFS analysis, was preserved,
suggesting that the treatment benefit of the subsequent therapies was not compromised by palbociclib + letrozole
PAL + LET
EFS = 38.8 months
PBO + LET
EFS = 28.8 months








