Data cutoff: February 26, 2016
a
ITT population;
b
Investigator assessment
CI, confidence interval; HR, hazard ratio; ITT, intention to treat; LET, letrozole; PAL, palbociclib; PBO, placebo; PFS,
progression-free survival
Finn RS, et al. Poster presented at ASCO 2017 (Abstract 1039)
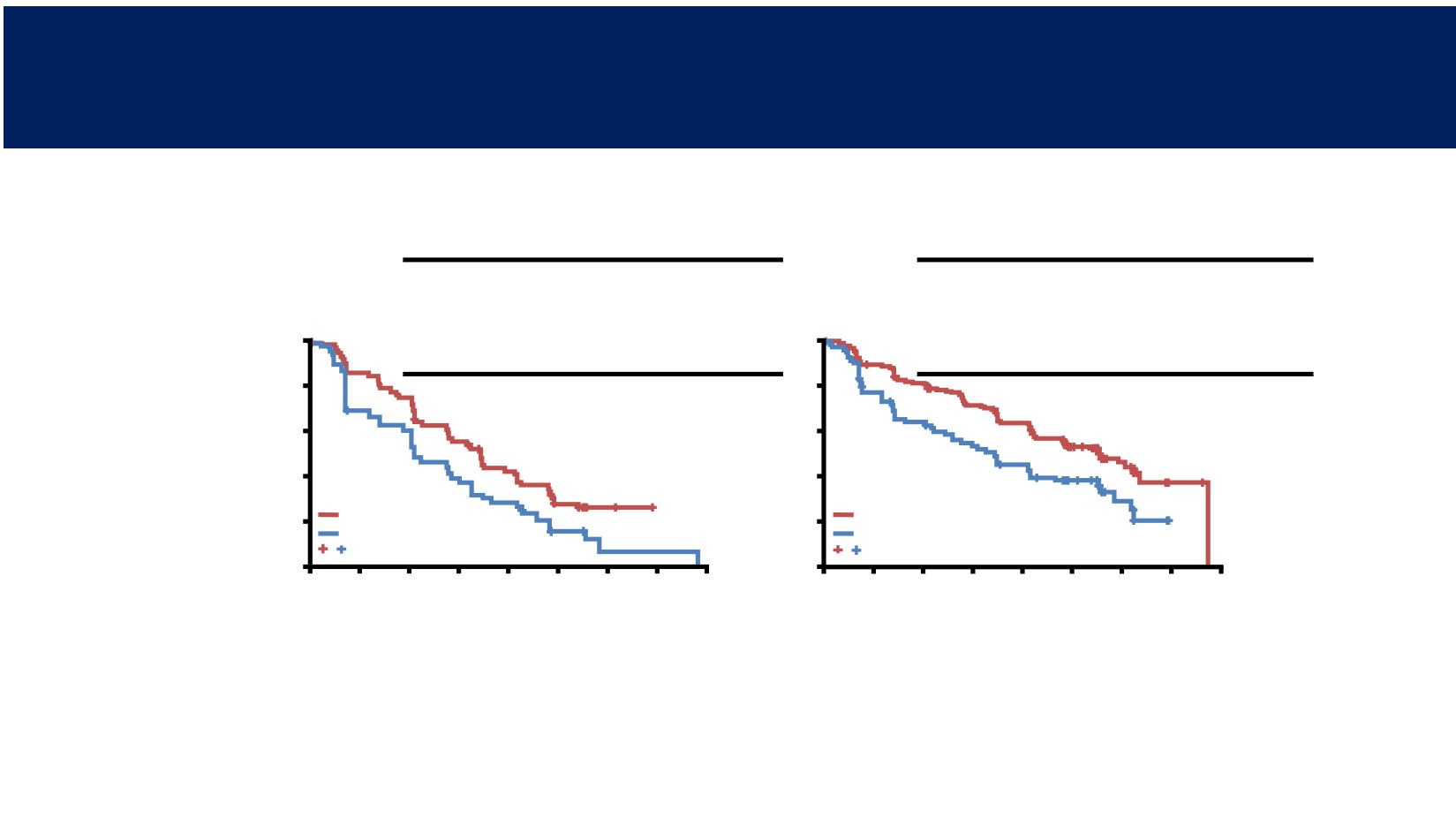
Liver involvement
Lung involvement – including pleura
PAL + LET
(n=75)
PBO + LET
(n=46)
Median PFS,
months (95% CI)
13.7
(10.9–16.6)
8.4
(5.5–12.9)
HR (95% CI)
0.62 (0.41–0.95)
P value
<0.05
PAL + LET
(n=171)
PBO + LET
(n=84)
Median PFS,
months (95% CI)
22.2
(16.8–25.4)
13.6
(8.4–18.5)
HR (95% CI)
0.59 (0.41–0.83)
P value
<0.01
PAL + LET
PBO + LET
No. at risk
75 62 54 39 28 14 2
0
46 30 26 16 12
5
1
1
0
PAL + LET
PBO + LET
No. at risk
171 148 131 114 101 57 22 4
84 60 49 40 33 21 8
0
Time (months)
0 4 8 12 16 20 24 28 32
PFS (%)
0
20
40
60
80
100
PBO + LET
Censored
PAL + LET
Time (months)
0 4 8 12 16 20 24 28 32
PFS (%)
0
20
40
60
80
100
PBO + LET
Censored
PAL + LET
Enfermedad Luminal
Agresiva
PFS in Patients with Liver and Lung Involvement








