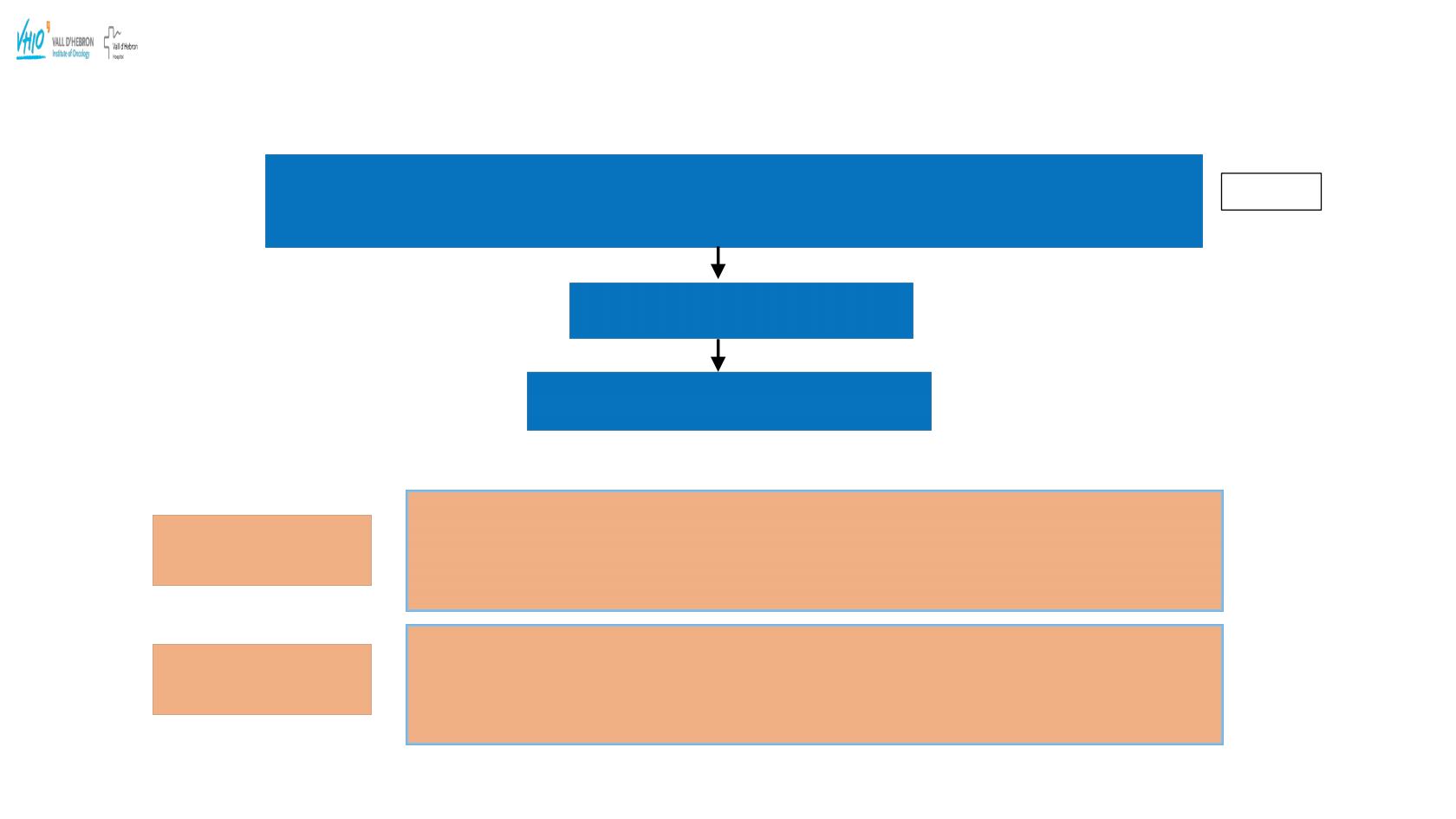
QUADRA: Single-Arm, Open-Label, Phase 2 Trial in Serous Epithelial Ovarian, Fallopian
Tube or Primary Peritoneal Cancer
clinicaltrials.gov: NCT02354586.
Endpoint assessment
Objective response rate (ORR) per RECIST in the
fourth and fifth-line HRD
positive patients
who were PARP inhibitor naïve, and platinum sensitive.
Primary Endpoint
Niraparib
300 mg daily, 28-day cycles
Patients with histologically diagnosed, advanced, relapsed, high-grade serous epithelial
ovarian, fallopian tube, or primary peritoneal cancer who have received 3 or more previous
chemotherapy regimens
(N=461)
Moore KN, et al. J Clin Oncol 2018;36(Suppl 15):Abstract 5514
Secondary Endpoints
Durability of response, disease control rate, progression free survival (PFS), overall survival
(OS) and safety and tolerability.








