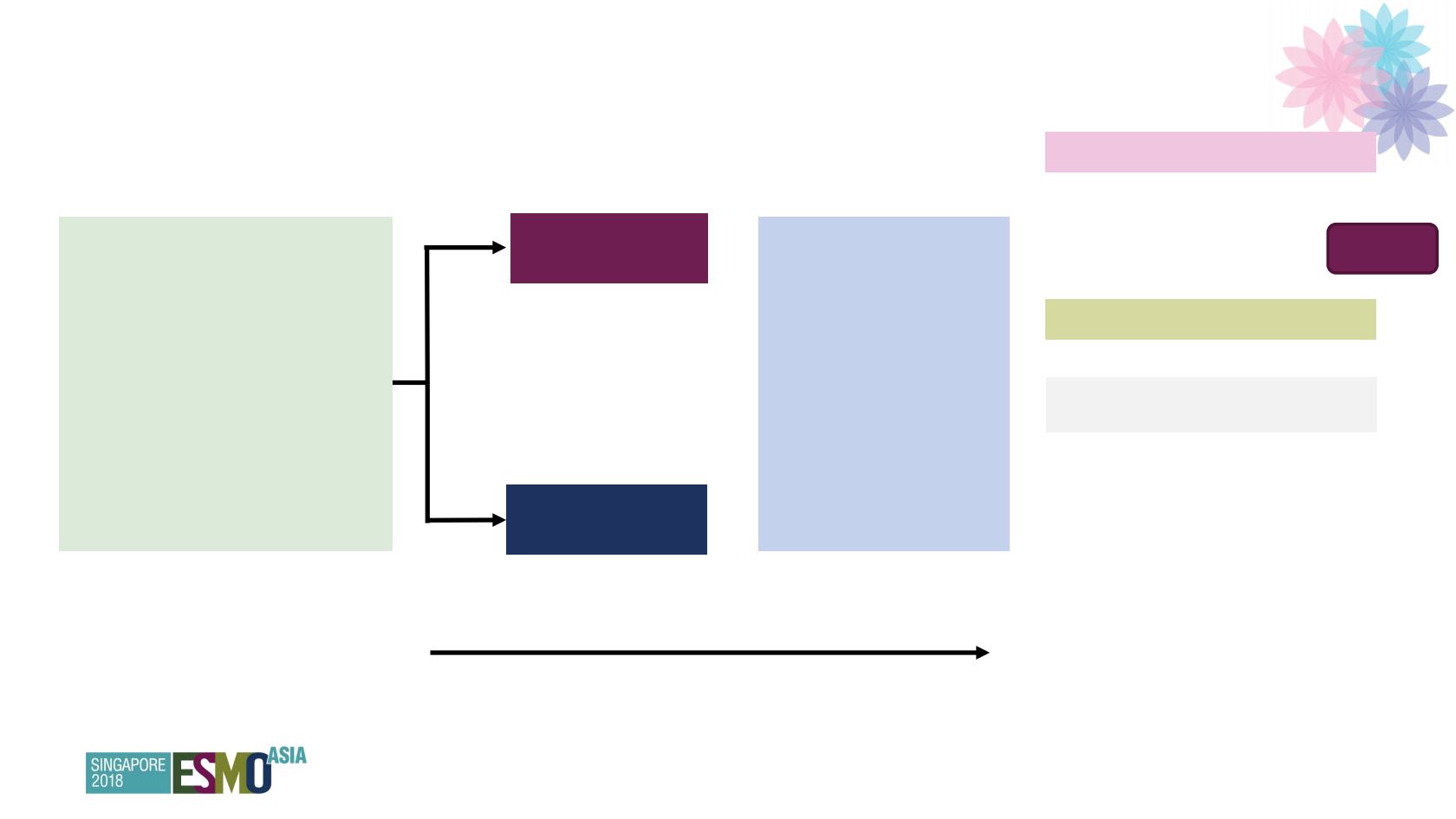
SOLO-1: Study design
*Upfront or interval attempt at optimal cytoreductive surgery for stage III disease and either biopsy and/or upfront or interval
cytoreductive surgery for stage IV disease. BICR, blinded independent central review; ECOG, Eastern Cooperative Oncology
Group; FACT-O, Functional Assessment of Cancer Therapy – Ovarian Cancer; FIGO, International Federation of Gynecology
and Obstetrics; HRQoL, health-related quality of life; PFS2, time to second progression or death;
RECIST, Response Evaluation Criteria in Solid Tumours; TOI, Trial Outcome Index
•
Newly diagnosed, FIGO
stage III–IV, high-grade serous
or endometrioid ovarian,
primary peritoneal or fallopian
tube cancer
•
Germline or somatic BRCAm
•
ECOG performance status 0–1
•
Cytoreductive surgery*
•
In clinical complete response or
partial response after platinum-
based chemotherapy
Olaparib 300 mg bd
(N=260)
Placebo
(N=131)
2:1 randomization
•
Study treatment
continued until
disease progression
•
Patients with no
evidence of disease at
2 years stopped
treatment
•
Patients with a partial
response at 2 years
could continue
treatment
Primary endpoint
•
Investigator-assessed PFS
(modified RECIST 1.1)
Secondary endpoints
•
PFS using BICR
•
PFS2
•
Overall survival
•
Time from randomization to
first subsequent therapy or
death
•
Time from randomization to
second subsequent therapy
or death
•
HRQoL (FACT-O TOI score)
Stratified by response
to platinum-based
chemotherapy
2 years’ treatment if no evidence of disease
HR 0.62








