Platinum combination followed by iPARP
Olaparib
study-19 design and results
Primary end point : PFS
Olaparib
400 mg po
bid
Randomized 1:1
Placebo
po bid
•
Platinum-sensitive high-grade
serous ovarian cancer
•
³
2 previous platinum regimens
•
Last chemotherapy was
platinum-based to which they
had a maintained PR or CR
prior to enrolment
•
Stable CA-125
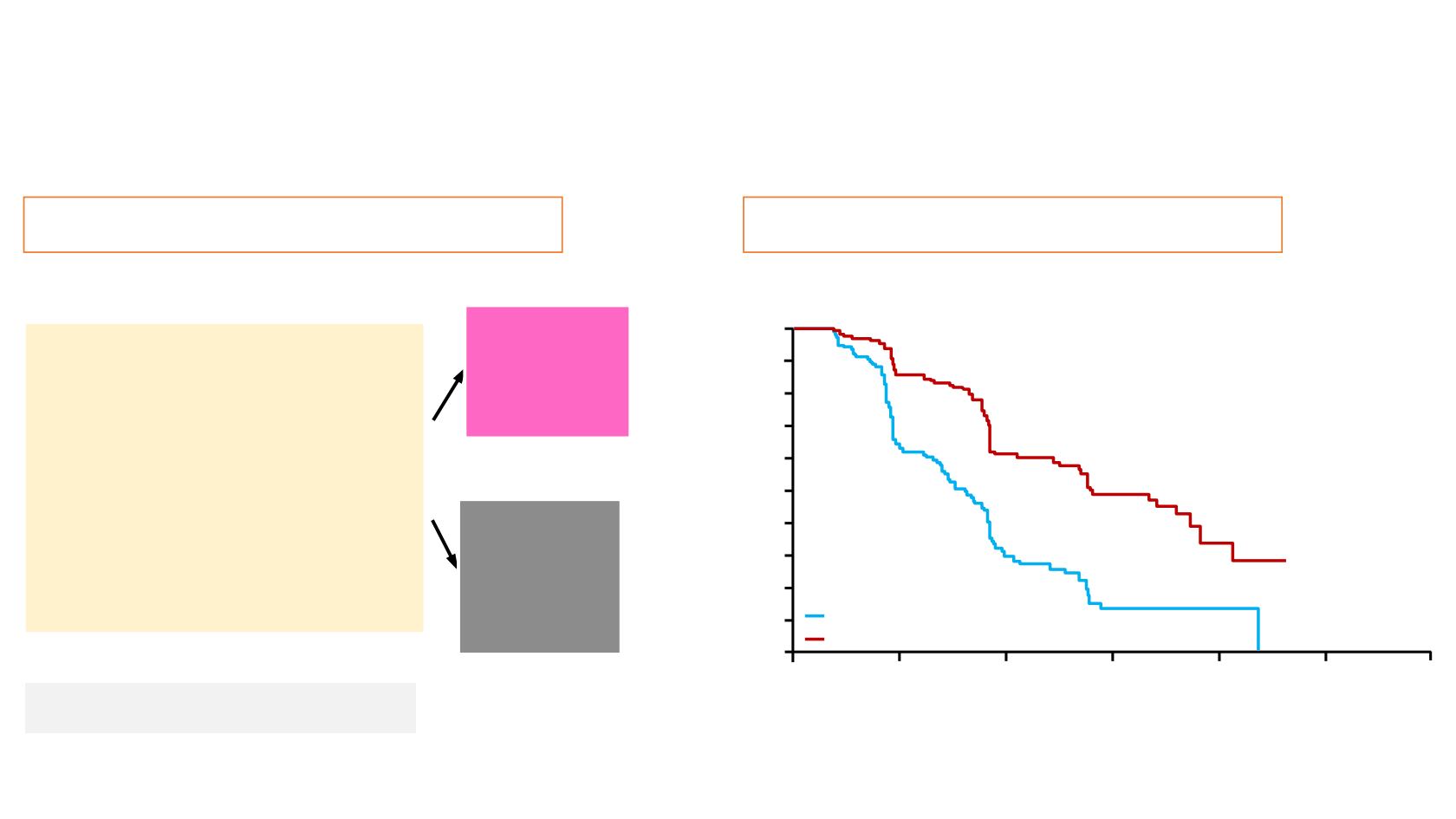
Study-19 aim and design
265 patients
Ledermann J, et al. N Engl J Med 2012;366:1382–92
Study-19 PFS
0
0.6
0.8
0.9
0
0.1
0.2
0.3
0.4
0.5
0.7
1.0
3
6
9
12
15
18
Probability of
progression-free survival
Time from randomization (months)
Hazard ratio 0.35,
(95% CI, 0.25–0.49);
P
<0.00001
Randomized treatment
Placebo
Olaparib 400 mg bid monotherapy
8.4 mos
4.8 mos








