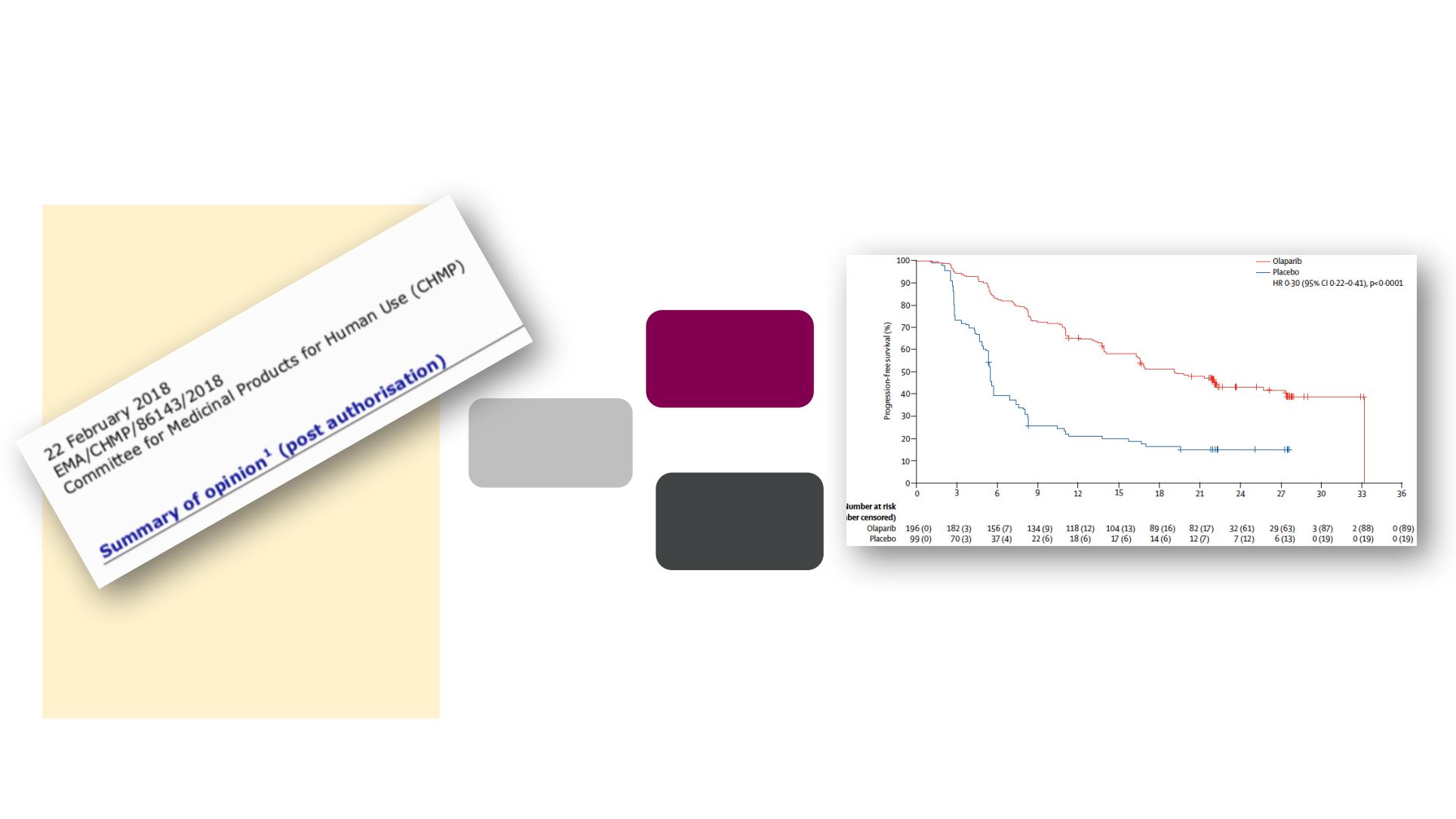
SOLO-2 is a randomised Phase 3 study of maintenance
olaparib in patients with BRCAm PSR OC
Olaparib
(n=196)
300mg
**
po
bid
Placebo
(n=99)
Randomise within
8 weeks of last
chemotherapy dose
Randomise 2:1
N=295
•
Stratification by response to
previous platinum-based
chemotherapy, time to disease
progression in penultimate
platinum-based chemotherapy
Pujade-Lauraine et al.
Lancet Oncol.
2017;18:1274-1284
•
Platinum-sensitive high-grade
serous or endometrioid
ovarian cancer
•
³
2 previous platinum regimens
•
Last chemotherapy was
platinum-based to which they
had a maintained PR or CR
prior to enrolment
•
Documented BRCAm
, or
patient willing to consent to
testing (only those with
deleterious or suspected
deleterious germline mutations
will be randomised)
19.1 vs 5.5 months
HR 0.3 (95% CI: 0.22-0.41)








