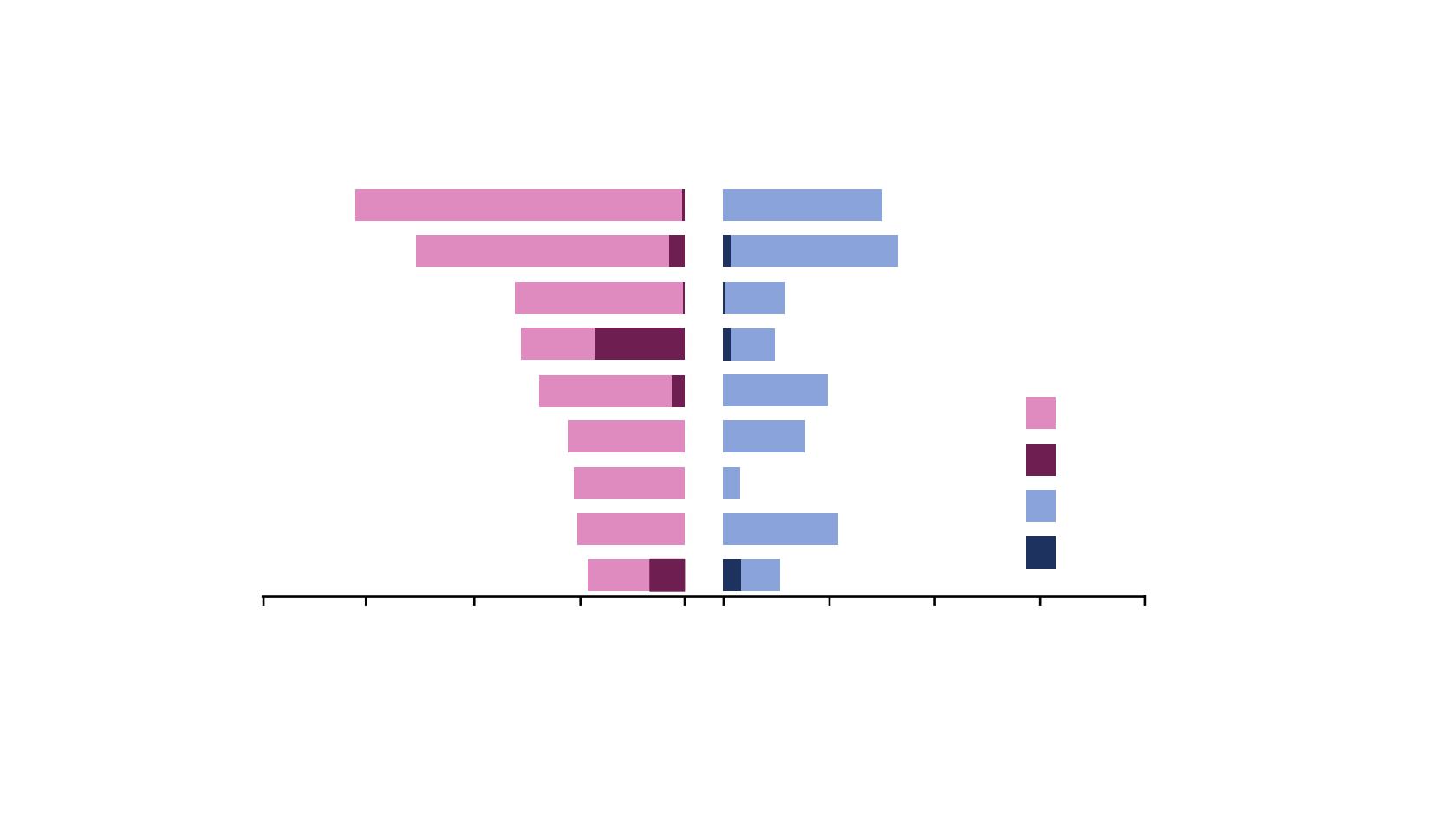
*Grouped terms. All-grade thrombocytopenia (grouped term) occurred in 11.2% of patients in the olaparib group and 3.8% of
patients in the placebo group and grade ≥3 thrombocytopenia (grouped term) occurred in 0.8% and 1.5%, respectively
Most common treatment-emergent adverse events
0.8
1.5
1.5
4.6
0.8
3.8
0.4
21.5
3.1
8.5
All grades (frequency ≥25%)
Grade ≥3 (frequency ≥5%)
All grades (frequency ≥25%)
Grade ≥3 (frequency ≥5%)
Placebo (N=130)
Olaparib (N=260)
23.1
25.4
26.2
27.7
34.2
38.8
40.0
63.5
77.3
Nausea
Fatigue/asthenia*
Vomiting
Anaemia*
Diarrhoe
a
Constipation
Dysgeusia
Arthralgia
Neutropenia*
11.5
26.9
3.8
19.2
24.6
10
41.5
37.7
14.6
100
75
50
25
0 0
25
50
75
100
Adverse events (%)








