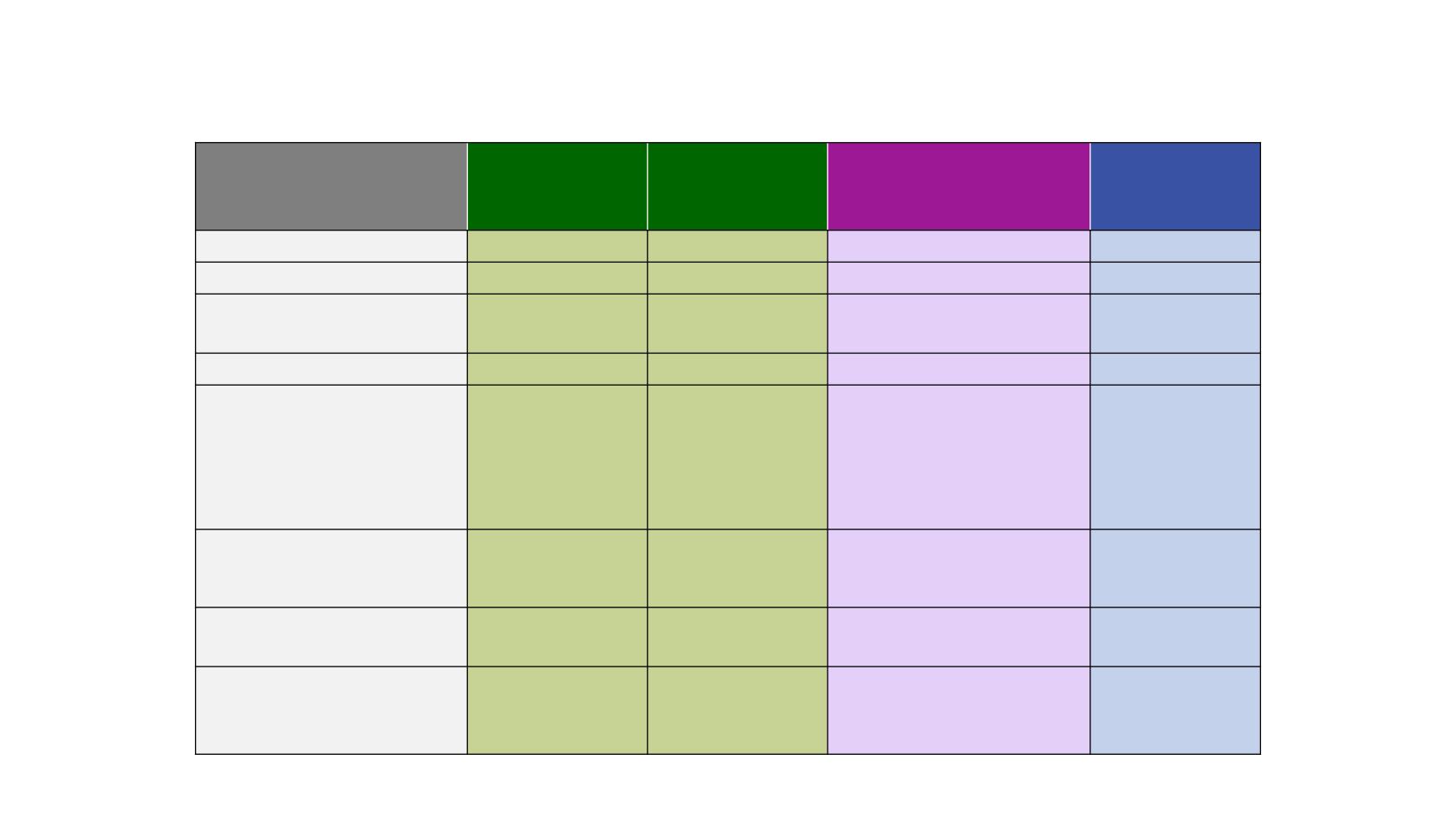
IO Phase III Trials in pre-treated patients
*850 in primary population
NR = not reached
1. Borghaei, et al. ASCO 2016
2. Herbst, et al. Lancet 2015; 3. Barlesi, et al. ESMO 2016
CheckMate 017
1
Nivolumab
vs docetaxel
CheckMate 057
1
Nivolumab
vs docetaxel
KEYNOTE-010
2
Pembrolizumab (2mg/kg or
10mg/kg) vs docetaxel
OAK
3
Atezolizumab
vs docetaxel
Phase of study
III
III
II/III
III
PD-L1 selected
No
No
Yes (TPS* ≥1%)
No
Study size, n
272
(135 vs 137)
582
(292 vs 290)
1,033
(344 vs 346 vs 343)
1,225
(425 vs 425)*
Histology
Squamous
Non-squamous
All-comers
All-comers
Line of therapy, %
2L
3L
>3L
Other/unknown
100
0
0
0
88
11
<1
0
69
20
9
<1
75
25
0
0
Subsequent CIT
(immunotherapy arm vs
chemo arm), %
<1 vs 2
1 vs 2
0.6 vs 1.7 vs 13.1
4.5 vs 17.2
Crossover from chemo arm to
study immunotherapy, %
4
6
Not permitted
Not permitted
Median OS, months
HR vs docetaxel (p value)
9.2 vs 6.0
0.62 (p=0.0004)
12.2 vs 9.5
0.75 (p<0.001)
10.4 vs 12.7 vs 8.5
2mg/kg: 0.71 (p=0.0008)
10mg/kg: 0.61 (p<0.0001)
13.8 vs 9.6
0.73 (p=0.0003)








