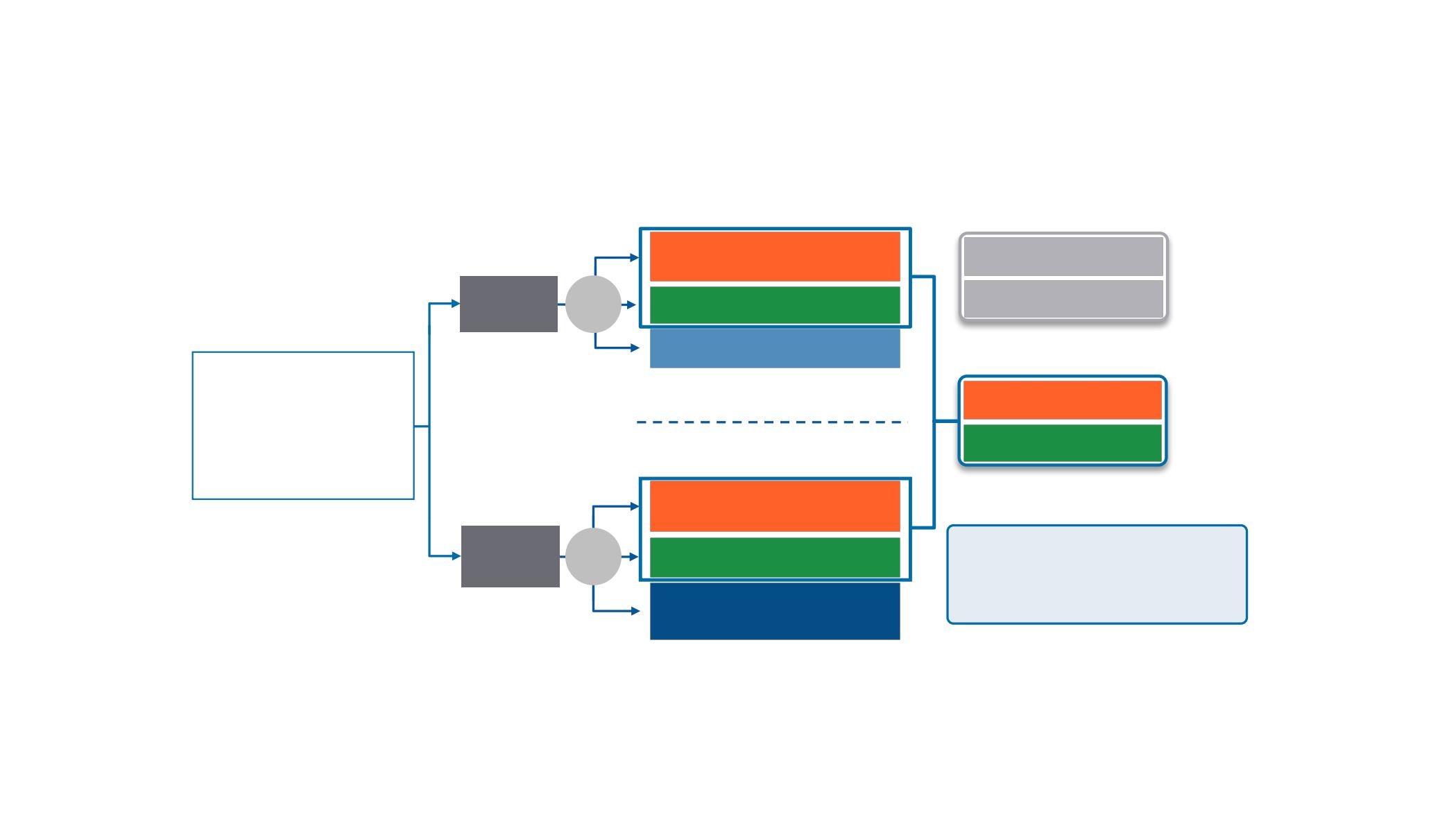
CheckMate 227 Part 1 Study Design
a
Database lock: January 24, 2018; minimum follow-up: 11.2 months
N = 1189
<1% PD-L1
expression
N = 550
Nivolumab 3 mg/kg Q2W
Ipilimumab 1 mg/kg Q6W
n = 396
Histology-based chemotherapy
b
n = 397
Nivolumab 240 mg Q2W
n = 396
Nivolumab 3 mg/kg Q2W
Ipilimumab 1 mg/kg Q6W
n = 187
Histology-based chemotherapy
b
n = 186
Nivolumab 360 mg Q3W +
histology-based chemotherapy
b
n = 177
R
1:1:1
Key Eligibility Criteria
•Stage IV or recurrent NSCLC
•No prior systemic therapy
•No known sensitizing
EGFR
/
ALK
alterations
•ECOG PS 0–1
Stratified by SQ vs NSQ
R
1:1:1
7
a
NCT02477826
b
NSQ:
pemetrexed + cisplatin or carboplatin, Q3W for ≤4 cycles, with optional pemetrexed maintenance following chemotherapy or nivolumab + pemetrexed maintenance
following nivolumab + chemotherapy;
SQ:
gemcitabine + cisplatin, or gemcitabine + carboplatin, Q3W for ≤4 cycles;
c
The TMB co-primary analysis was conducted in the subset of patients
randomized to nivolumab + ipilimumab or chemotherapy who had evaluable TMB ≥10 mut/Mb
≥1% PD-L1
expression
Nivolumab + ipilimumab
n = 396
Chemotherapy
b
n = 397
Patients for PD-L1 co-primary analysis
Co-primary endpoints:
Nivolumab +
ipilimumab vs chemotherapy
•
OS in PD-L1–selected populations
• PFS in TMB-selected populations
Nivolumab + ipilimumab
n = 139
Chemotherapy
b
n = 160
Patients for TMB co-primary analysis
c
Hellmann et al., NEJM 2018








