Post-treatment
Follow-up
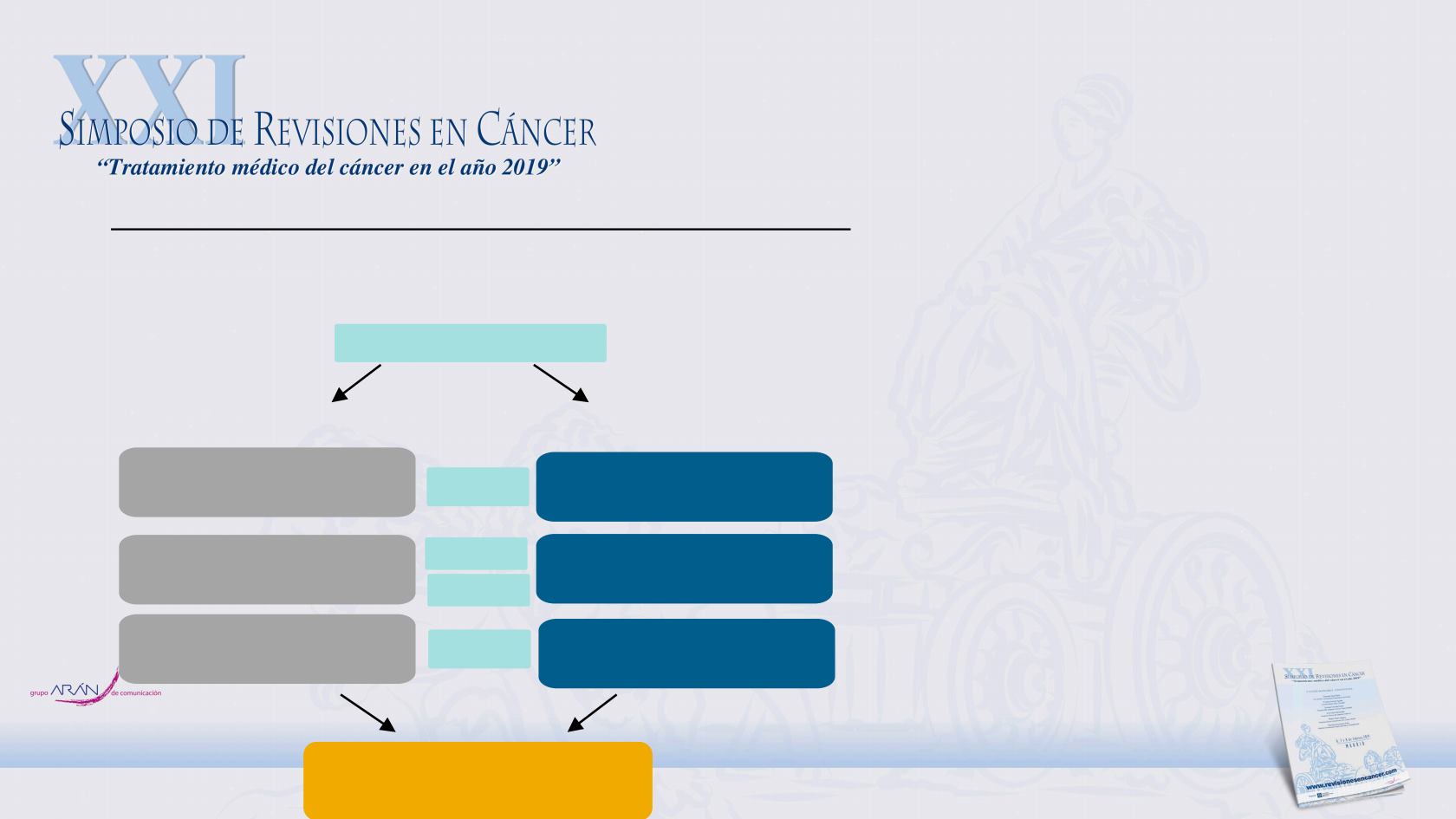
REGISTRATION
Carboplatin AUC6 D1
Paclitaxel 200mg/m
2
D1
Pembrolizumab 200mg D1
Cisplatin 75 mg/m
2
D1
Pemetrexed 500 mg/m
2
D1
Pembrolizumab 200mg D1
Cohort A (any histology)
Cohort B (non-squamous only)
Carboplatin AUC2 D1, D8, D15
Paclitaxel 45mg/m
2
D1, D8, D15
Pembrolizumab 200mg D1
Pembrolizumab 200mg D1
Cisplatin 75 mg/m
2
D1
Pemetrexed 500 mg/m
2
D1
Pembrolizumab 200mg D1
Pembrolizumab 200mg D1
CYCLE 1
Radiotherapy
CYCLE 4-17
CYCLE 2-3
ClinicalTrials.gov Identifier: NCT03631784
End Points
•
Primary:
Grade 3 or higher pneumonitis.
ORR by BICR (Blinded Independent Central
Review
)
•
Secondary: PFS (RECIST v1.1 per blinded,
independent central review), OS, Safety
Beneficio y toxicidad de IO + QT-RT concomitante
•
KEYNOTE-799 phase II trial CT+ Pembrolizumab + RT. NCT03631784
Dudas en CPNM estadio
III irresecable








