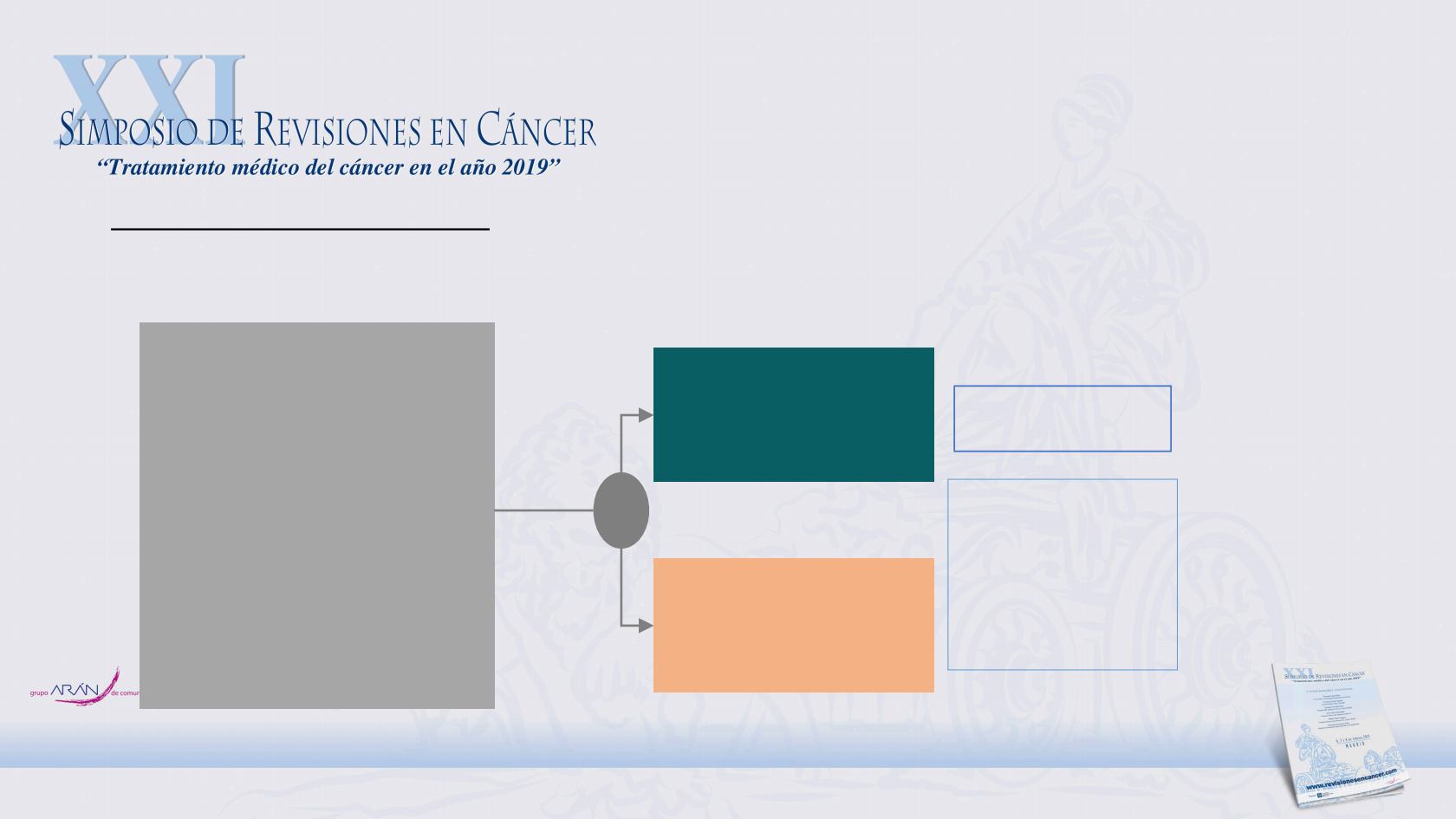
Beneficio de IO tras SBRT
•
PACIFIC 4 Phase 3 trial: NTC03833154
• Stage I/II lymph-node
Negative Non-small Cell
Lung Cancer without
progression after SBRT
• Histo/citollogically
documented
• 18 years or older
• WHO PS score 0 or 2
• PD-L1 known
N: 630 pts
DURVALUMAB 1500 mg
c/4s 24 months
Placebo c/4s 24 months
Key secondary
endpoint
• OS
Primary endpoint
• PFS
R
Dudas en CPNM estadio
III irresecable








