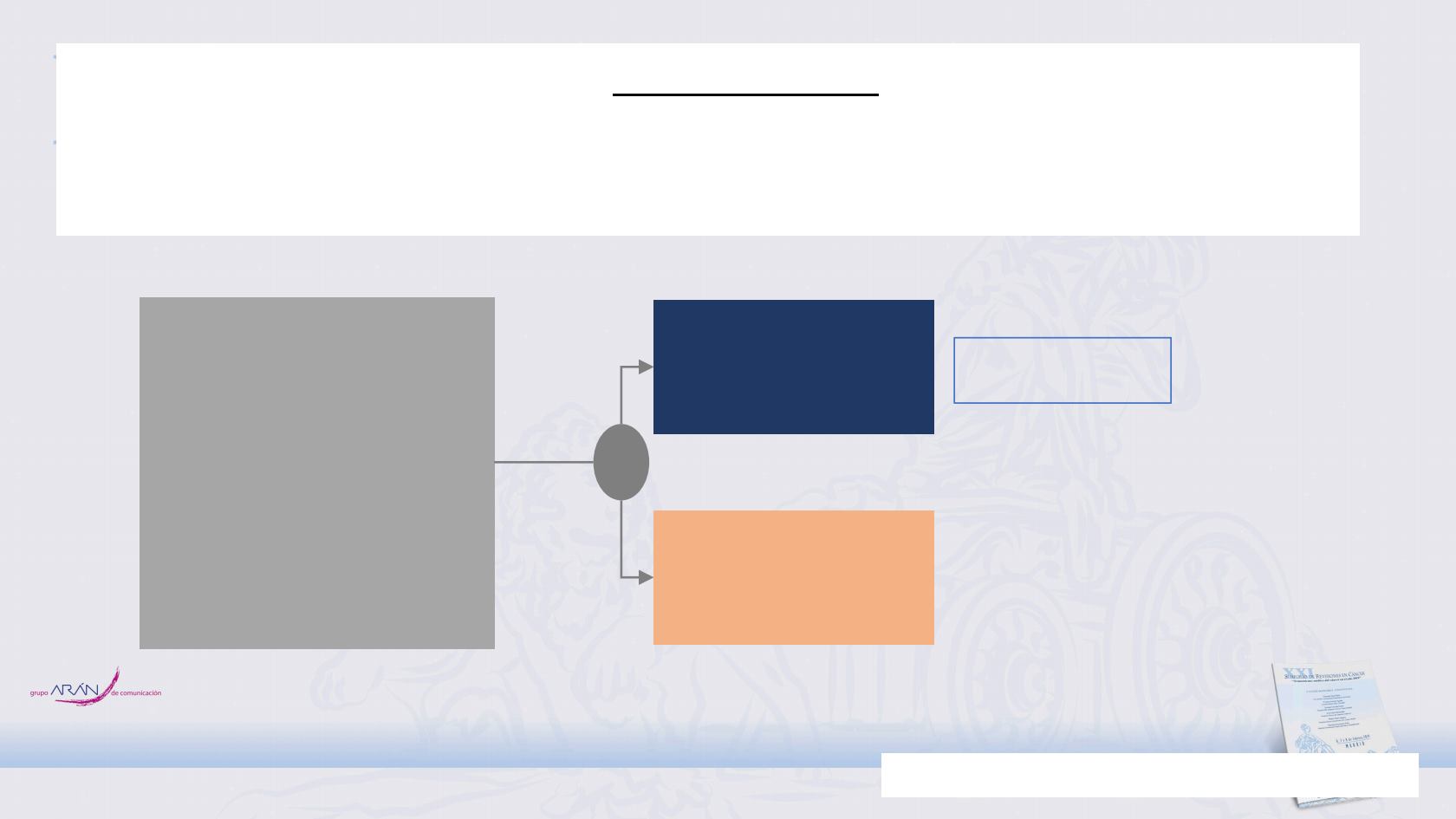
A Randomized Phase II Study of Pembrolizumab (MK-3475) as
Maintenance Therapy in Patients With Unresectable Stage III Non-small
Cell Lung Cancer Treated With Definitive Chemo-radiotherapy
ClinicalTrials.gov Identifier: NCT03379441
Prof. Silvia Novello, Principal Investigator, University of Turin, Italy
• Unresectable, Stage IIIA-B
NSCLC without
progression after definitive
platinum-based cCRT
(sequential/concurrent)
• 18 years or older
• WHO PS score 0 or 1
• If available, archived pre-
cCRT tumor tissue
N: 126 pts
Pembrolizumab
200 mg q3w for
up to 24 months
OBSERVATION
Key secondary
endpoint
• PFS: Rate (%) of
patients without
disease
progression at
12, 18 and 24
Primary endpoint
• OS
R








