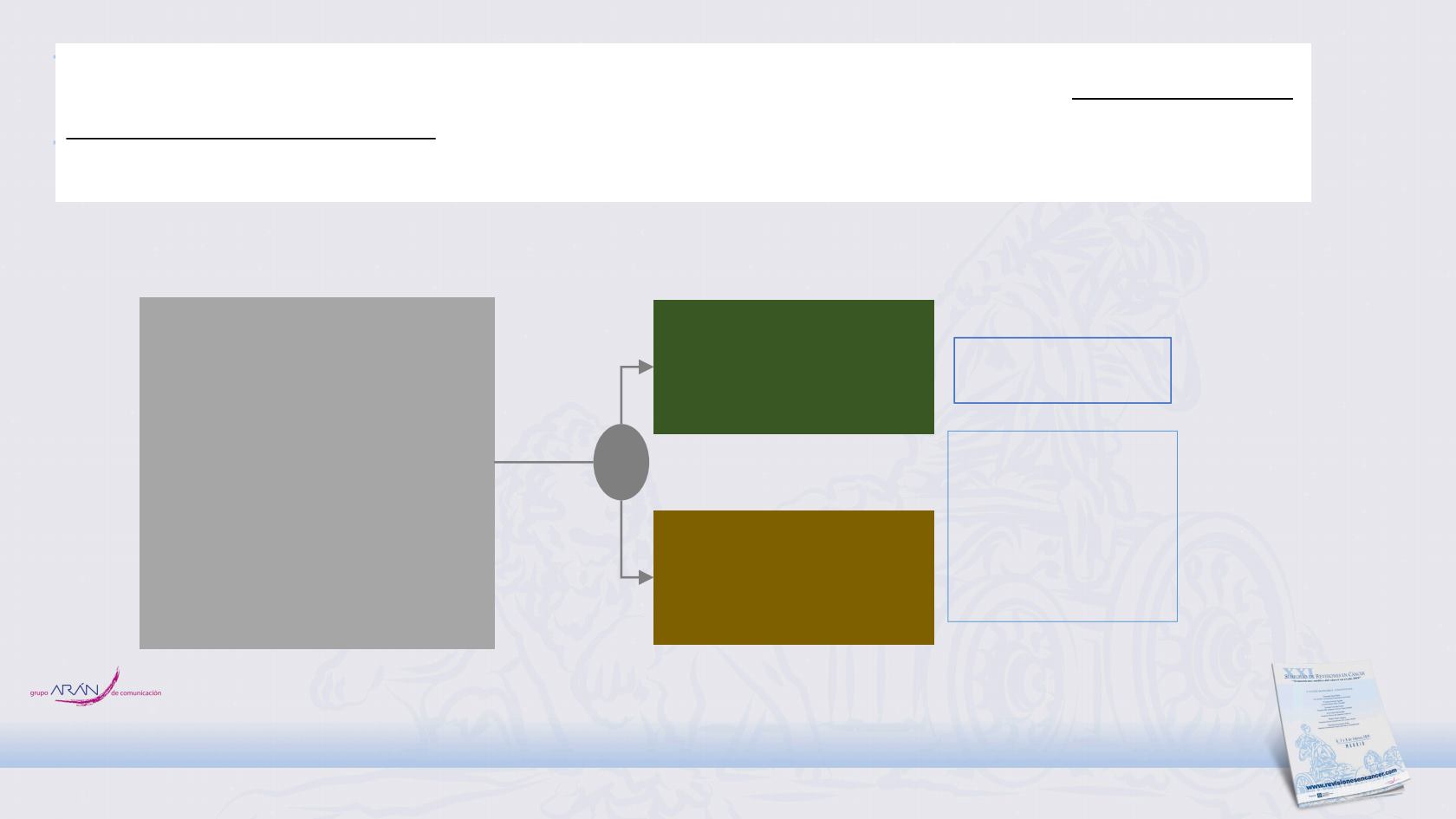
NCT03285321: Phase II Study of Consolidation Immunotherapy With Nivolumab and
Ipilimumab or Nivolumab Alone Following Concurrent Chemoradiotherapy for
Unresectable Stage IIIA/IIIB Non-small Cell Lung Cancer (NSCLC): BTCRC-LUN16-081
• Unresectable, Stage IIIA-B
NSCLC without
progression after definitive
platinum-based
CONCURRENT CRT
• 18 years or older
• WHO PS score 0 or 1
• PD-L1 known
N:108
Nivolumab 480 mg c/4w
6 cycles
Nivolumab 3mg/kg c/2w
12 doses + IPI 1 mg/kg
c/6w 4 cycles
Key secondary
endpoint
• OS
• TTMD
• AEs
Primary endpoint
• PFS
R








