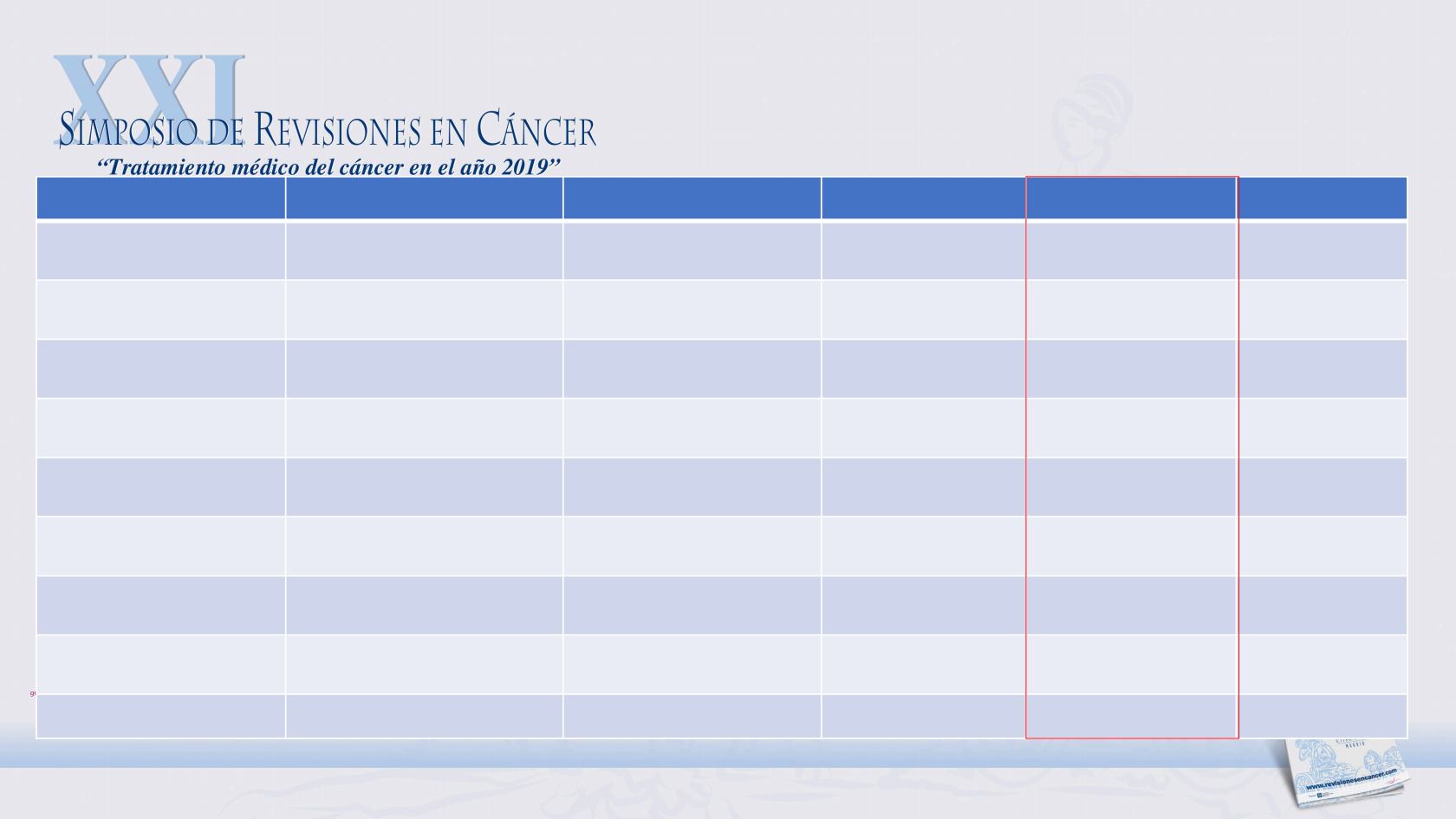
AGENT/TRIAL
STATUS
CONTROL ARM
OS (MONTHS)
HAZARD RATIO
P VALUE
SIPULEUCEL-T
IMPACT
QT naïve
Placebo
25.8
0.78
0.03
ABIRATERONE-P
COU-AA-302
QT naïve
Prednisone
34.7
0.81
0.0033
ENZALUTAMIDE
PREVAIL
QT naïve
Placebo
32.4
0.70
<0.0001
DOCETAXEL-P
TAX-327
QT naïve
Mitoxantrone-P
18.9
0.76
0.009
ABIRATERONE-P
COU-AA-301
Post-docetaxel
Prednisone
15.8
0.74
<0.0001
ENZALUTAMIDE
AFFIRM
Post-docetaxel
Placebo
18.4
0.63
<0.0001
CABAZITAXEL-P
TROPIC
Post-docetaxel
Mitoxantrone-P
15.1
0.70
<0.0001
RADIUM 223
ALSYMPCA
Pre&Post-docetaxel
Placebo
14.9
0.70
<0.001
DENOSUMAB*
Bone mets
Zoledronic
20.7*
0.82
0.008
PHASE III TRIALS IN METASTATIC CASTRATION-RESISTANT PROSTATE CANCER
Kantoff PW, et al. N Engl J Med 2010;363:411-22. Ryan CJ, et al. Lancet Oncol 2015;16(2):152-60. Tannock IF, et al.N Engl J Med 2004;351(15):1502-12.
De Bono JS, et al. Lancet 2010;376:1147-54. Fizazi K, et al. Lancet Oncol 2012;13(10):983-92. Scher HI, et al. N Engl J Med 2012;367(13):1187-97.
Parker C, et al. N Engl J Med 2013;369:213-23. Fizazi K, et al. Lancet 2011;377(9768):813-22. Beer TM, et al. N Engl J Med 2014;371(5):424-33.








