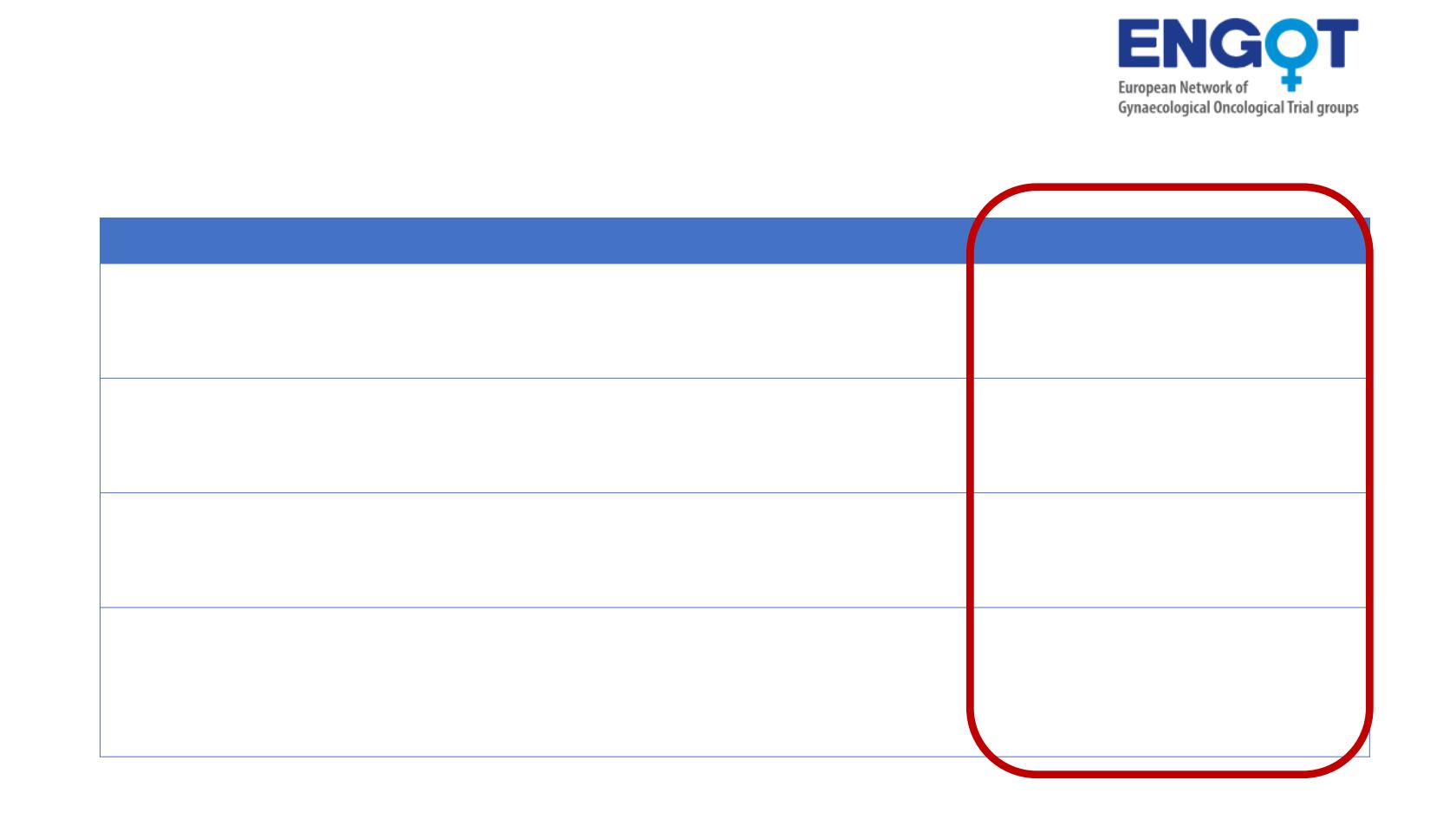
Front line for Stage III/IV with PARPi / IO / Bev
TRIAL
Setting
Patient selection
Arms
AGO / DUO-O ENGOT
Ov46
Front line
tBRCAnon-mut*
PDS or IDS Any residual
LGSOC excluded
CP-Bev-placebo-placebo
CP-Bev-Durvalumab-placebo
CP-Bev-Durvalumab-Olaparib
BGOG /ENGOT Ov43 Front line
tBRCA non-mut*, Any histotype
PDS or IDS Any residual
Bev optional
CP-Placebo-Placebo
CP- Pembro-Placebo
CP- Pembro-Olaparib
GINECO/ FIRST
ENGOT Ov44
Front line
PDS (high risk) or IDS
Bev optional
Mucinous excluded
CP-Placebo-Placebo
CP-Placebo-Niraparib
CP-TSR042-Niraparib
ATHENA
GOG3020 / ENGOT
Ov45
Maintenance
after front line
Stage III-IV and high grade
PDS or IDS
Response to platinum
Rucaparib-Nivolumab
Rucaparib-Placebo
Nivolumab-Placebo
Placebo-Placebo
* Separate clinical trial for tBRCA-mutated








