HRD=homologous recombination deficiency; CR=complete response; PR=partial response; PFS=progression-free
survival;
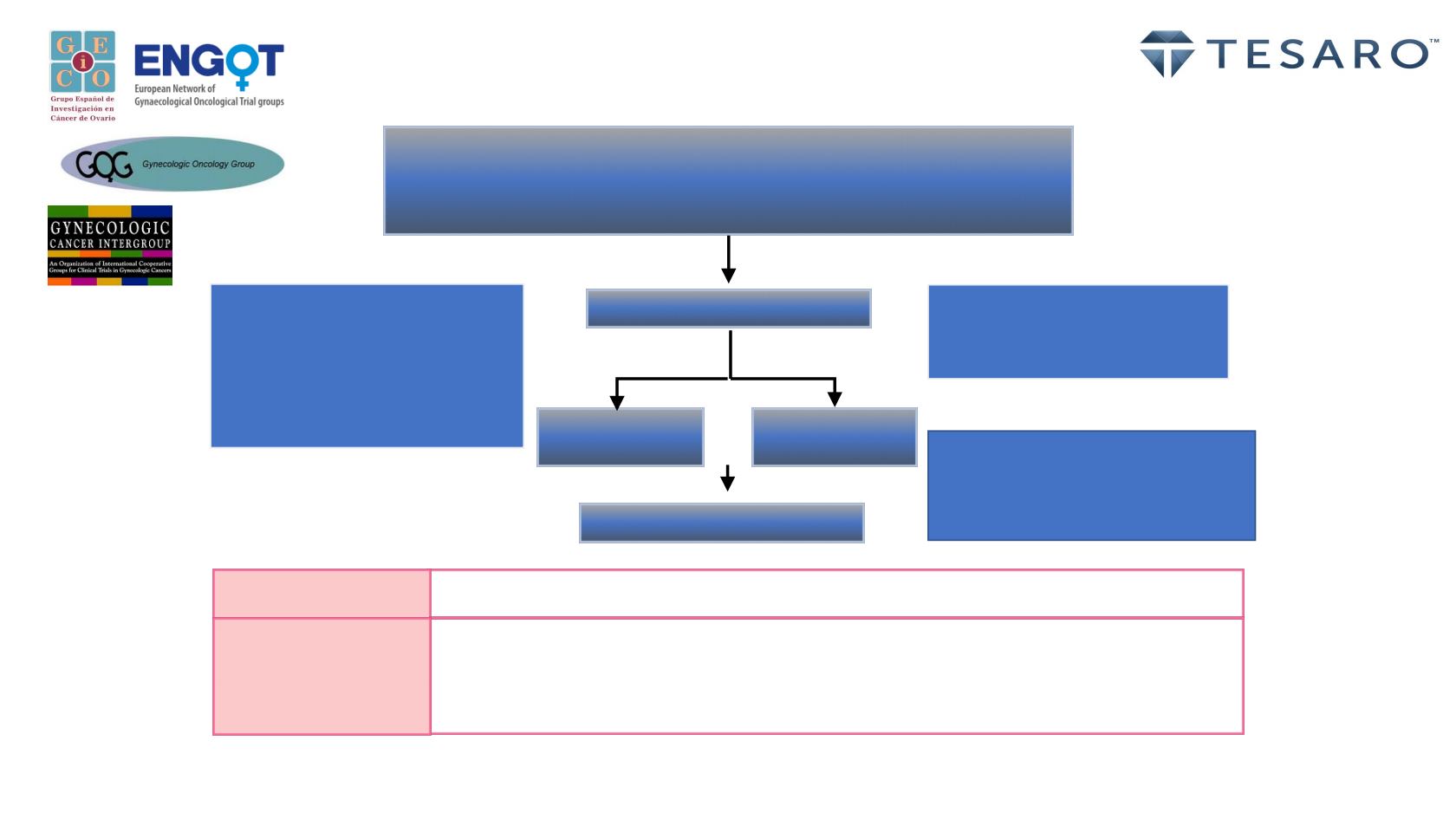
ENGOT ov26 / PRIMA Study
Endpoint assessment
Niraparib
300 mg
Placebo
Platinum responsive ovarian cancer
Stage III or IV ovarian
CR or PR with front line platinum-based chemotherapy
HRDpos or HRDneg/not determined (nd) tumor
2:1 Randomization
PFS in HRDpos patients; hierarchical analysis for all patients regardless of HRD
status
Primary Endpoint
Overall survival (OS), patient reported outcomes (PRO’s), time to first
subsequent treatment, progression- survival-2 , time to CA-125 progression,
safety and tolerability of study therapy
Key Secondary
Endpoints
pre-enrollment screening:
•
centralized HRD testing for
all patients
•
local sBRCA and/or gBRCA
test results are allowed
Stratification factors:
•
Use of NACT: yes or no
•
Best tumor response: CR or PR
•
HRD status: pos or neg/nd
• Patients with sBRAC or tBRCAmut
will be stratified as HRDpos
• Patients with unknown or wild type
BRCA will be stratified based on
HRD test results
CONFIDENTIAL








