Clinical Therapeutics/Volume 38, Number 7, 2016
A Randomized Phase I Pharmacokinetic Study
Comparing Biosimilar Candidate SB3 and
Trastuzumab in Healthy Male Subjects
Xavier Pivot, MD, PhD
1
; Elsa Curtit, MD
1
; Yoon Jung Lee, PhD
2
; George Golor, MD
3
;
Anke Gauliard, MD
3
; Donghoon Shin, MD, PhD
2
; Youngdoe Kim, MS
2
;
Hansook Kim, MS
2
; and Rainard Fuhr, MD, PhD
3
1
University Hospital Jean Minjoz, INSERM 1098, Besancon, France;
2
Samsung Bioepis Co Ltd, Incheon,
Republic of Korea; and
3
PAREXEL International Early Phase Clinical Unit, Berlin, Germany
ABSTRACT
Purpose:
This
fi
rst-in-human study with SB3 was
designed to evaluate the pharmacokinetic (PK) equivalence
between SB3 and trastuzumab sourced in the European
Union (EU trastuzumab), between SB3 and trastuzumab
sourced in the United States (US trastuzumab), and
between EU and US trastuzumab (NCT02075073).
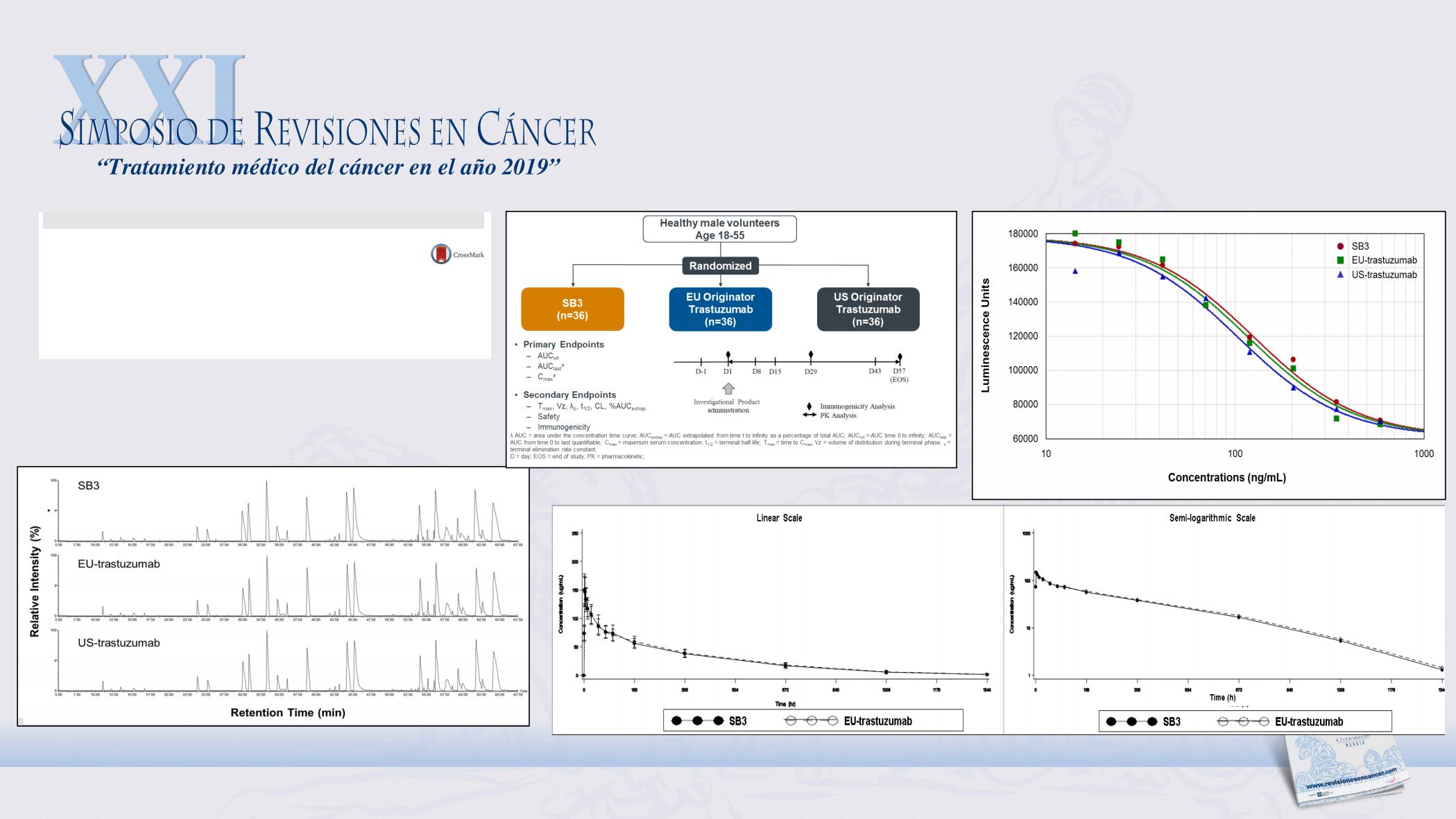
Methods:
In this randomized, double-blind, parallel
group, single-dose comparative PK study, 109 healthy
male subjects were randomized to receive a single 6-
mg/kg IV dose of SB3, EU -trastuzumab, or US
trastuzumab. The PK parameters were calculated
using noncompartmental methods. The PK equiva-
lence in terms of AUC
0-
–
1
)
, AUC
0
–
last
, and C
max
for
the pairwise comparisons (SB3 vs EU trastuzumab,
SB3 vs US trastuzumab, and EU trastuzumab vs US
trastuzumab) were determined using the prede
fi
ned
equivalence margin of 0.8 to 1.25.
Findings:
Baseline demographic characteristics for
the randomized subjects were similar across the 3
groups. The 90% CIs for the geometric least square
means of the AUC
0
–
1
, AUC
0
–
last
, and C
max
were
completely contained within the margin of 0.8 to
1.25. The proportions of subjects who experienced
adverse events related to the study drug were 36.1%,
44.4%, and 61.1% in the SB3, EU trastuzumab, and
US trastuzumab groups, respectively. The most fre-
quently reported adverse events related to the study
drug was infusion-related reactions. No subjects had
positive results for antidrug antibodies after a single
dose of SB3, EU trastuzumab, or US trastuzumab.
Implications:
This study revealed PK equivalence
between SB3 and EU trastuzumab, between SB3 and
US trastuzumab, and between EU trastuzumab and US
trastuzumab. SB3 is well tolerated without tolerability
concerns after single-dose administration in healthy
male subjects. (
Clin Ther.
2016;38:1665
–
1673)
&
2016 Elsevier HS Journals, Inc. All rights
reserved.
Key words:
biosimilars, breast cancer, Herceptin
s
,
pharmacokinetic equivalence, trastuzumab.
INTRODUCTION
Trastuzumab
*
is a humanized IgG1 monoclonal
antibody selectively targeting the HER2 protein that is
overexpressed in breast canecr cell. The mechanism of
action of trastuzumab is known to be its inhibition of
proliferation of human tumor cells that overexpress
HER2 and potent mediation of antibody-dependent
cell-mediated cytotoxicity.
1
–
4
Trastuzumab-containing
regimens are standard of care for the treatment of
HER2-positive breast cancer, providing signi
fi
cant clin-
ical bene
fi
t in the adjuvant setting for early breast
cancer
5
–
7
and in treatment of advanced or metastatic
breast cancer.
8
–
10
It is administered intravenously either
with a loading dose of 8 mg/kg and a maintenance dose
of 6 mg/kg on a 3-weekly basis or with a loading dose of
4 mg/kg and a maintenance dose of 2 mg/kg weekly.
11
A biosimilar is a biological medicinal product that
is highly similar to an already authorized original
biological medicinal product (reference medicinal
product) in terms of quality, tolerability, and ef
fi
cacy
Accepted for publication June 6, 2016.
http://dx.doi.org/10.1016/j.clinthera.2016.06.0020149-2918/$-see front matter
&
2016 Elsevier HS Journals, Inc. All rights reserved.
*
Trademark: Herceptin
s
(Hoffman La Roche Registration Ltd,
Basel, Switzerland).
First in Humans: Estructura FQ y
FC – FD similares al original








