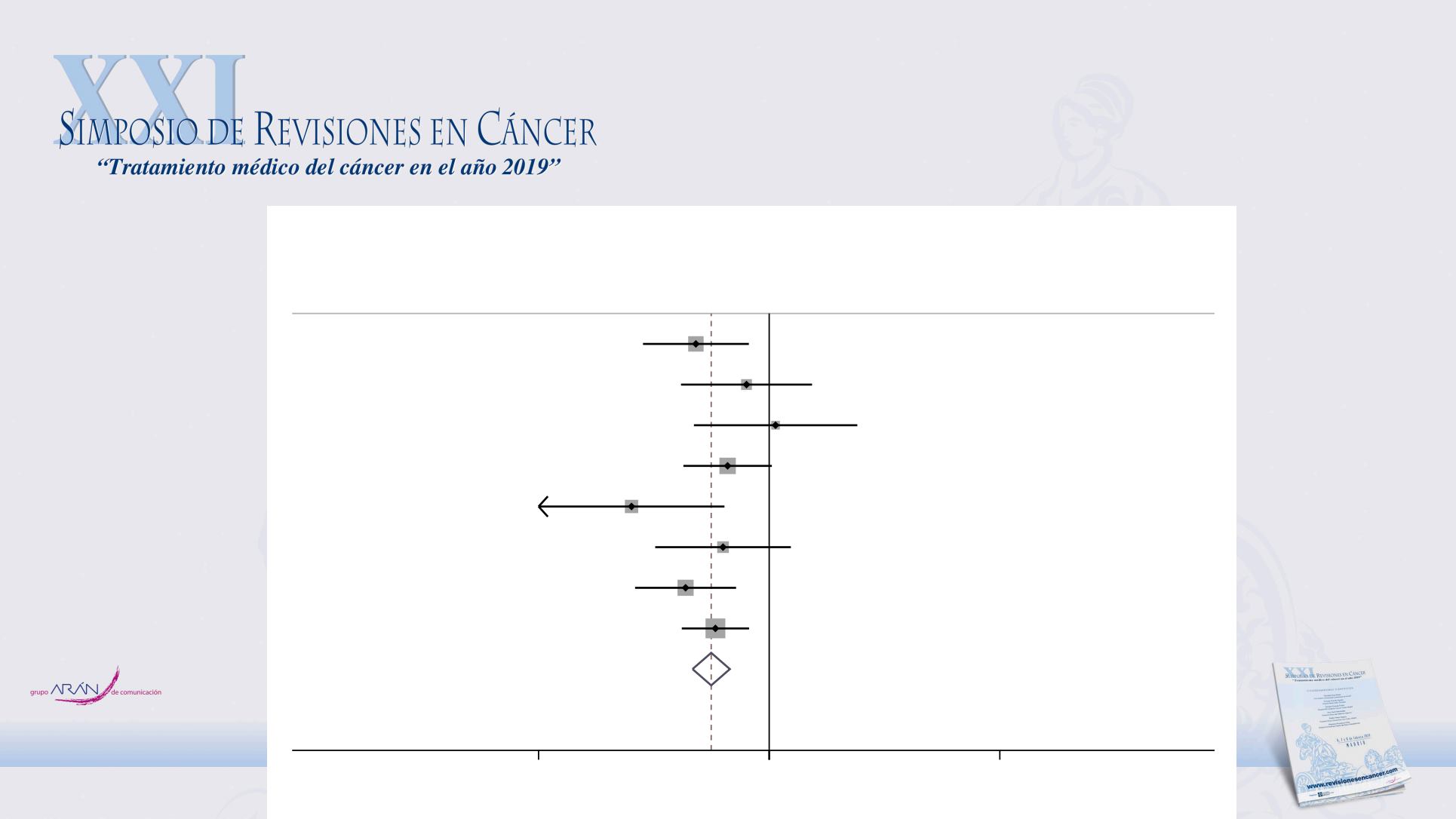
for immune checkpoint inhibitors in
urothelial carcinoma
Overall (I-squared = 16.1%, p = 0.304)
Sharma 2016 (Nivolumab)
Sharma 2017 (Nivolumab)
Patel 2018 (Avelumab)
ID
Rosenberg 2016 (Atezolizumab )
Powles 2017 (Durvalumab)
Balar 2017 (Pembrolizumab)
Balar 2016 (Atezolizumab )
Study
Petrylak 2018 (Atezolizumab )
0.53 (0.43, 0.65)
1.07 (0.44, 2.61)
0.64 (0.39, 1.03)
0.60 (0.29, 1.27)
RR (95% CI)
0.40 (0.23, 0.70)
0.22 (0.08, 0.61)
0.56 (0.39, 0.80)
0.78 (0.38, 1.60)
0.45 (0.25, 0.80)
100.00
3.60
16.67
7.48
Weight
15.95
10.25
25.47
6.16
%
14.43
1
.081
12.3
X. Rui et al.
,QWHUQDWLRQDO ,PPXQRSKDUPDFRORJ\
²
Rui, X et al. Int Immunopharmacol 2019








