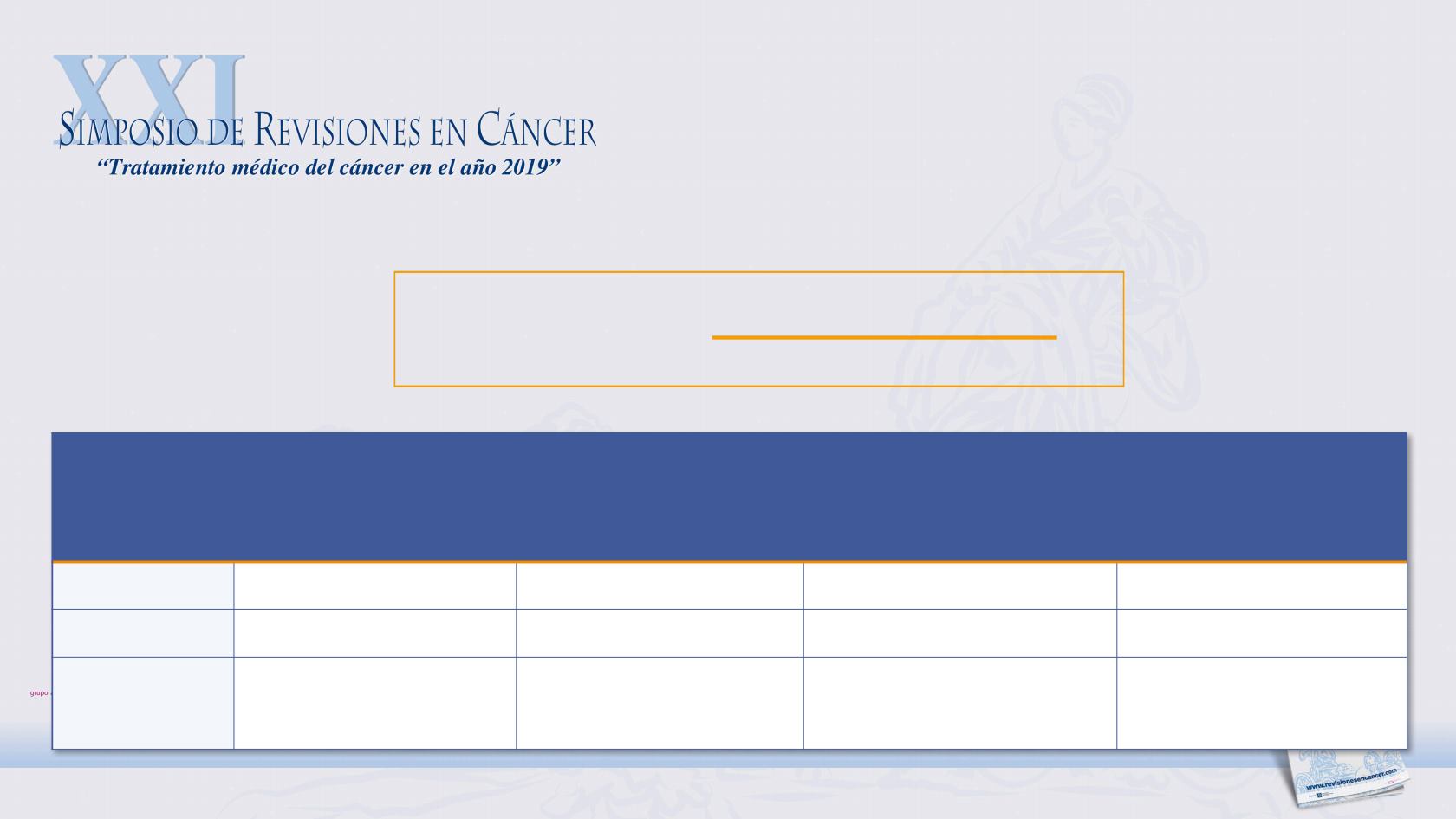
expression of PD-L1 appears to
be predictive for pembrolizumab
in several tumour types
3L+
gastric/GEJ
adenocarcinoma
2L+
cervical cancer
Cisplatin-ineligible
1L metastatic
urothelial carcinoma
1L
SCCHN
Threshold
CPS ≥1
CPS ≥1
CPS ≥10
CPS ≥20
Approval
US
US
EU, US
under investigation
Select
efficacy EP
ORR 13.3%
(95% CI: 8.2–20.0)
ORR 14.3%
(95% CI: 7.4–24.1)
ORR 47%
(95% CI: 38–57)
OS met
PFS not reached
Key tumour types where PD-L1 CPS appears to be predic:ve of response*
# PD-L1 staining cells
(tumour cells, lymphocytes, macrophages)
Combined positive score (CPS)
=
----------------------------------------------------
x 100
Total # viable tumour cells
KEYTRUDA [package insert]. Whitehouse Station, NJ: Merck & Co; 2018.
Merck.
http://investors.merck.com/news/press-release-details/2018/KEYTRUDA-pembrolizumab-Monotherapy-Met-a-Primary-Endpoint-in-the-Phase-3-KEYNOTE-048-Trial-Significantly-Improving-OS-as-First-Line-Therapy-in-Head-and-Neck-Squamous-Cell-Carcinoma-Patients-Whose-Tumors-Expressed-PD-L1-
CPS-20/default.aspx. July 25, 2018. Accessed July 25, 2018.






