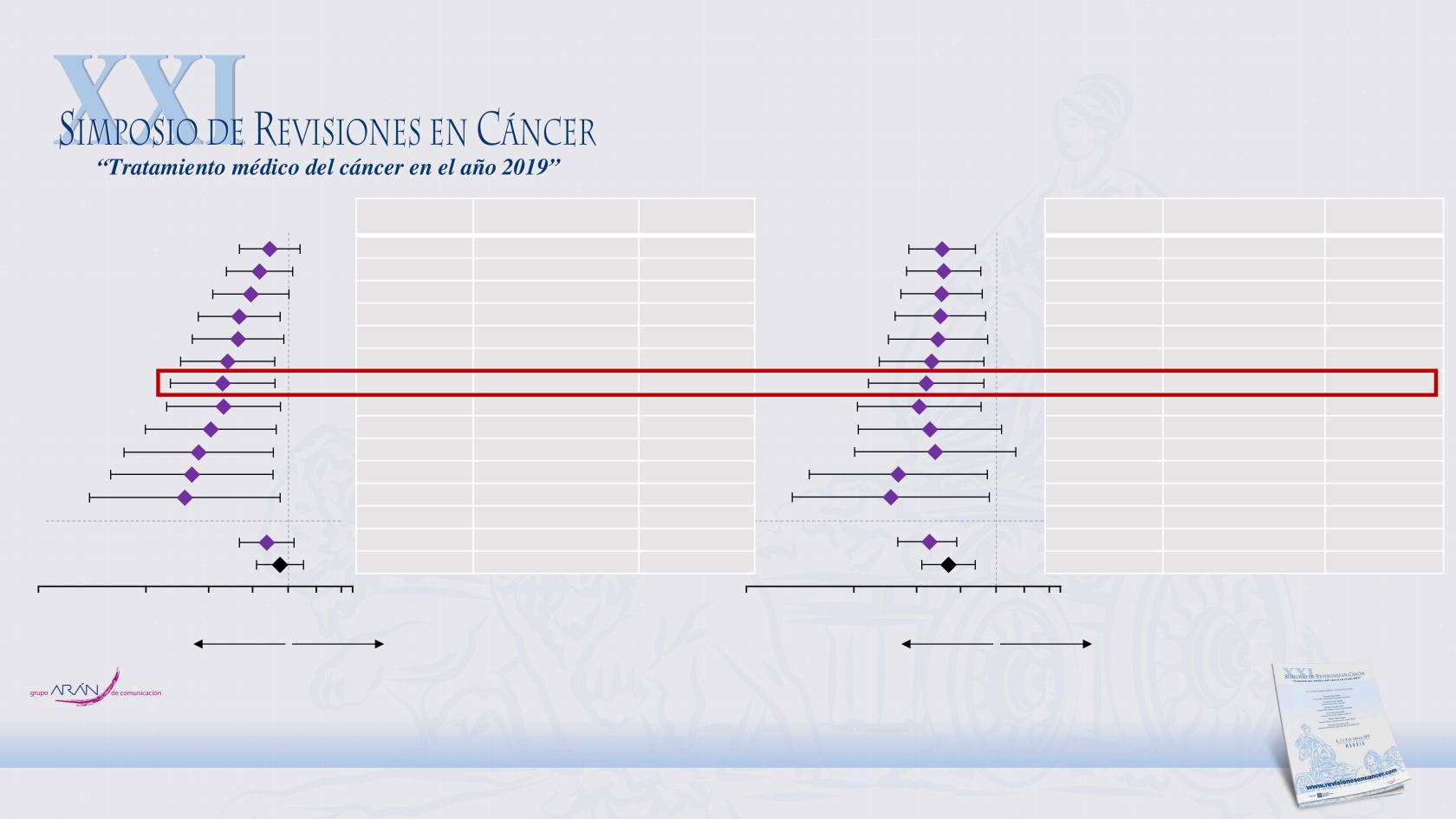
Population PFS HR (95% CI)
n (%)
bTMB ≥4
0.89 (0.73, 1.08)
441 (76%)
bTMB ≥6
0.83 (0.67, 1.03)
371 (64%)
bTMB ≥8
0.79 (0.62, 1.00)
302 (52%)
bTMB ≥10
0.73 (0.56, 0.95)
251 (43%)
bTMB ≥12
0.73 (0.54, 0.97)
211 (36%)
bTMB ≥14
0.68 (0.50, 0.92)
188 (32%)
bTMB ≥16
0.65 (0.47, 0.92)
158 (27%)
bTMB ≥18
0.66 (0.46, 0.95)
136 (23%)
bTMB ≥20
0.61 (0.40, 0.93)
105 (18%)
bTMB ≥22
0.57 (0.35, 0.91)
84 (14%)
bTMB ≥24
0.54 (0.32, 0.91)
69 (12%)
bTMB ≥26
0.51 (0.28, 0.95)
54 (9%)
BEP
0.87 (0.73, 1.04)
583 (100%)
ITT
0.95 (0.82, 1.10)
850
Population OS HR (95% CI)
n (%)
bTMB ≥4
0.70 (0.57, 0.87)
441 (76%)
bTMB ≥6
0.71 (0.56, 0.90)
371 (64%)
bTMB ≥8
0.70 (0.54, 0.91)
302 (52%)
bTMB ≥10
0.69 (0.52, 0.93)
251 (43%)
bTMB ≥12
0.68 (0.50, 0.94)
211 (36%)
bTMB ≥14
0.66 (0.47, 0.92)
188 (32%)
bTMB ≥16
0.64 (0.44, 0.92)
158 (27%)
bTMB ≥18
0.61 (0.41, 0.90)
136 (23%)
bTMB ≥20
0.65 (0.41, 1.03)
105 (18%)
bTMB ≥22
0.67 (0.40, 1.13)
84 (14%)
bTMB ≥24
0.53 (0.30, 0.94)
69 (12%)
bTMB ≥26
0.50 (0.27, 0.95)
54 (9%)
BEP
0.64 (0.53, 0.77)
583 (100%)
ITT
0.73 (0.62, 0.87)
850
•
Enrichment of PFS benefit was observed in the blood-based TMB (bTMB) ≥16 subgroup, while OS was consistent between the bTMB ≥16 subgroup and
the BEP
30
Subgroups in OAK
Progression-Free Survival
Overall Survival
0.2
1.0
HR
1.5
Favors atezolizumab Favors docetaxel
0.2
1.0
HR
1.5
Favors atezolizumab Favors docetaxel
BEP=biomarker-evaluable population.
Gandara DR et al.
Nat Med
. 2018;24(9):1441-1448.
Gandara et al., 2018,
Nat Med
.








