OS rate, % 1-year
2-year
Atezolizumab
+ CnP
63.1
39.6
CnP
55.5
30.0
Cappuzzo F, et al. Ann Oncol 2018;29(suppl 5):Abstr LBA53
PFS rate, % 6-month 12-month
Atezolizumab
+ CnP
56.1
29.1
CnP
42.5
14.1
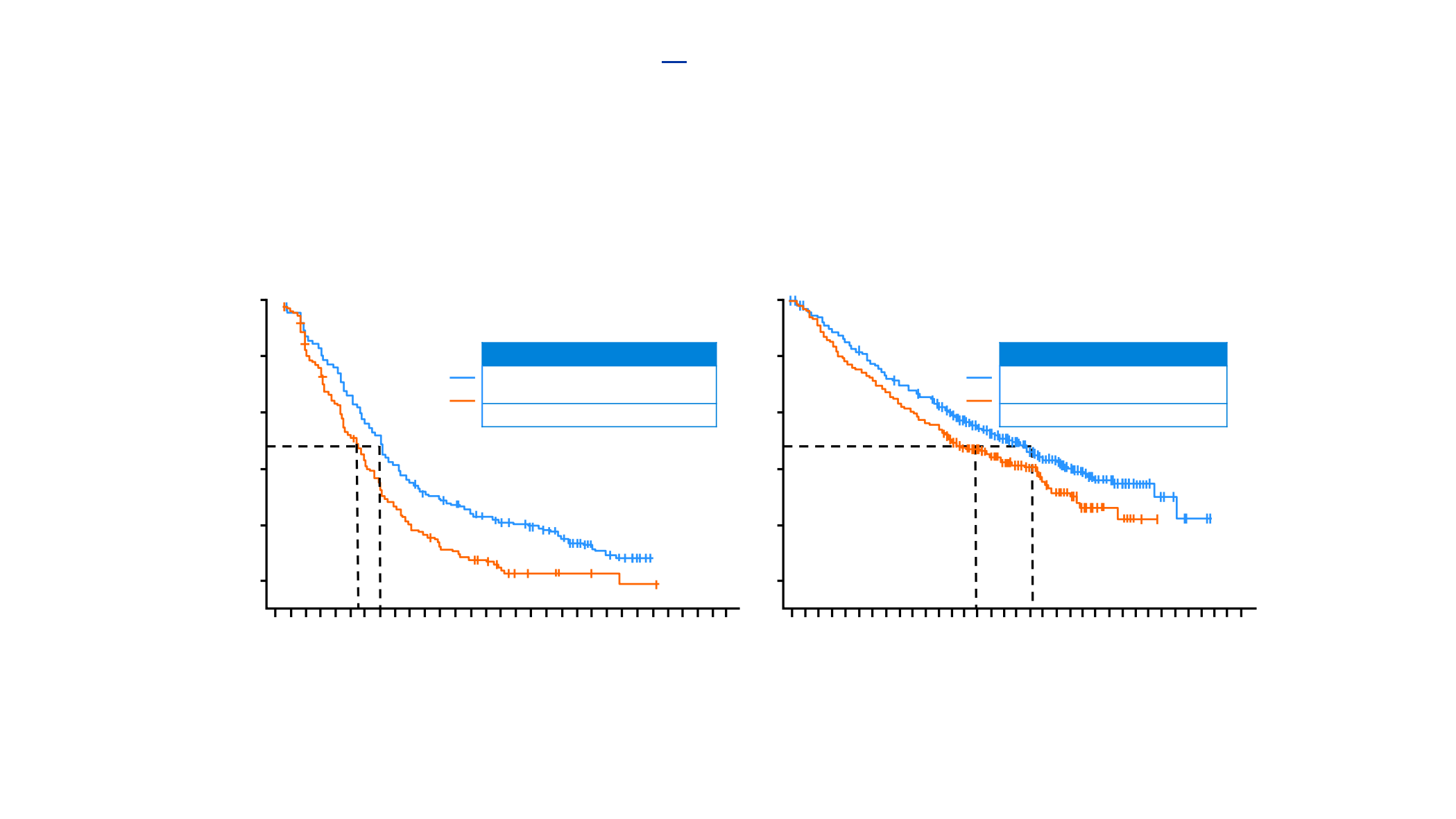
Investigator-assessed PFS (ITT-WT)
PFS, %
Months after randomisation
1.0
0.8
0.6
0.4
0.2
0.0
0
451
228
1
432
214
2
383
174
3
351
150
4
329
136
5
281
110
30
No. at risk
Atezo + CnP
Chemo
6
242
90
7
213
75
8
183
61
9
157
48
10
138
40
11
132
35
12
119
29
13
108
23
14
83
18
15
78
15
16
62
7
17
60
6
18
41
5
19
36
5
20
29
3
21
23
3
22
13
2
23
12
2
24
7
1
25
4
1
26
1
27 28 29
Median follow-up: ~19 months
HR 0.64
(95%CI 0.54, 0.77)
p<0.0001
OS (ITT-WT)
OS, %
Months after randomisation
1.0
0.8
0.6
0.4
0.2
0.0
0
451
228
1
435
218
2
422
206
3
400
190
4
384
176
5
365
167
30
4
No. at risk
Atezo + CnP
Chemo
6
351
161
7
333
154
8
315
147
9
305
136
10
294
132
11
284
124
12
268
119
13
253
109
14
217
96
15
194
90
16
167
75
17
147
65
18
129
58
19
103
49
20
88
39
21
75
31
31
2
22
59
24
32
1
23
49
17
33
24
40
13
34
25
29
9
26
19
8
27
12
3
28
10
1
29
6
HR 0.79
(95%CI 0.64, 0.98)
p=0.033
Median: 5.5 mo
(95%CI 4.4, 5.9)
Median: 7.0 mo
(95%CI 6.2, 7.3)
Median: 13.9 mo
(95%CI 12.0, 18.7)
Median: 18.6 mo
(95%CI 16.0, 21.2)
Nab-Paclitaxel/Carbo+ Atezo in Non-SCC NSCLC
Impower 130 Trial








