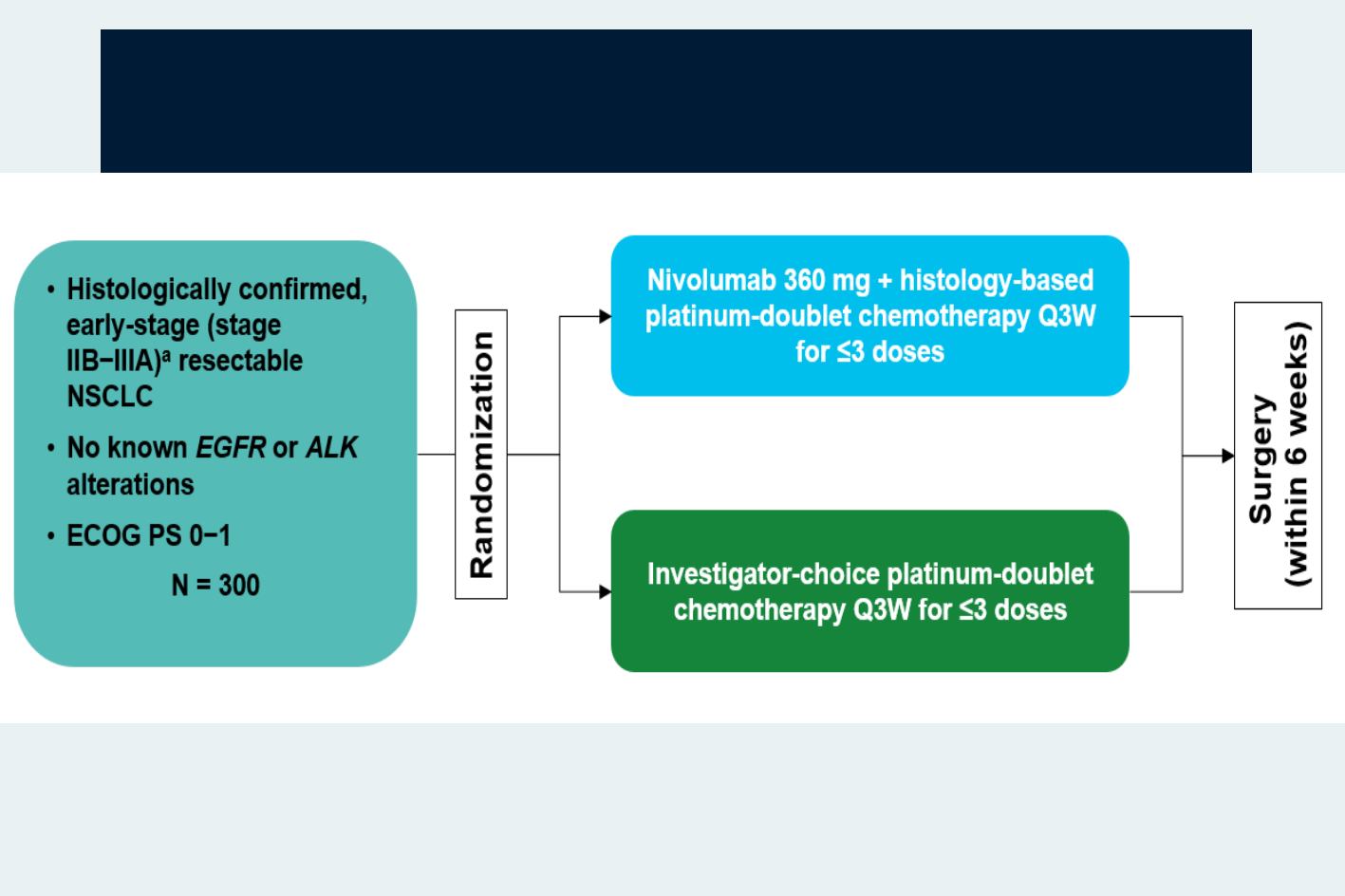
CheckMate 816: Phase 3 Neoadjuvant Trial of Nivolumab and
Chemotherapy
4
9
Co-primary endpoints -
EFS and pCR rate (assessed by blinded independent review) with nivolumab plus platinum-doublet
chemotherapy vs platinum-doublet chemotherapy
Secondary endpoints -
MPR rate, OS, Time to death or distant metastases








