The results of the PROSPER trial were recently
published in the
New England Journal of
Medicine
1
.This multicentre, double-blind,
phase III trial randomized 1,401 men with
nonmetastatic castration-resistant prostate
cancer (nmCRPC) and a prostate-specific
antigen doubling time (PSADT) of<10months
in a 2:1 ratio to receive enzalutamide or pla-
cebo. The first interim analysis after 447 events
demonstrated significantly improved metas-
tasis-free survival (MFS) in the enzaluta-
mide group compared with the control group
(36.6 months versus 16.2 months; HR 0.29;
95% CI 0.24–0.35;
P
<0.001)
1
. Analysis of the
secondary end points demonstrated signif-
icant improvement in median time to PSA
progression (37.2 months versus 3.9 months;
also an
and fu
by the
tamide
for n
similar
gressio
confir
direct
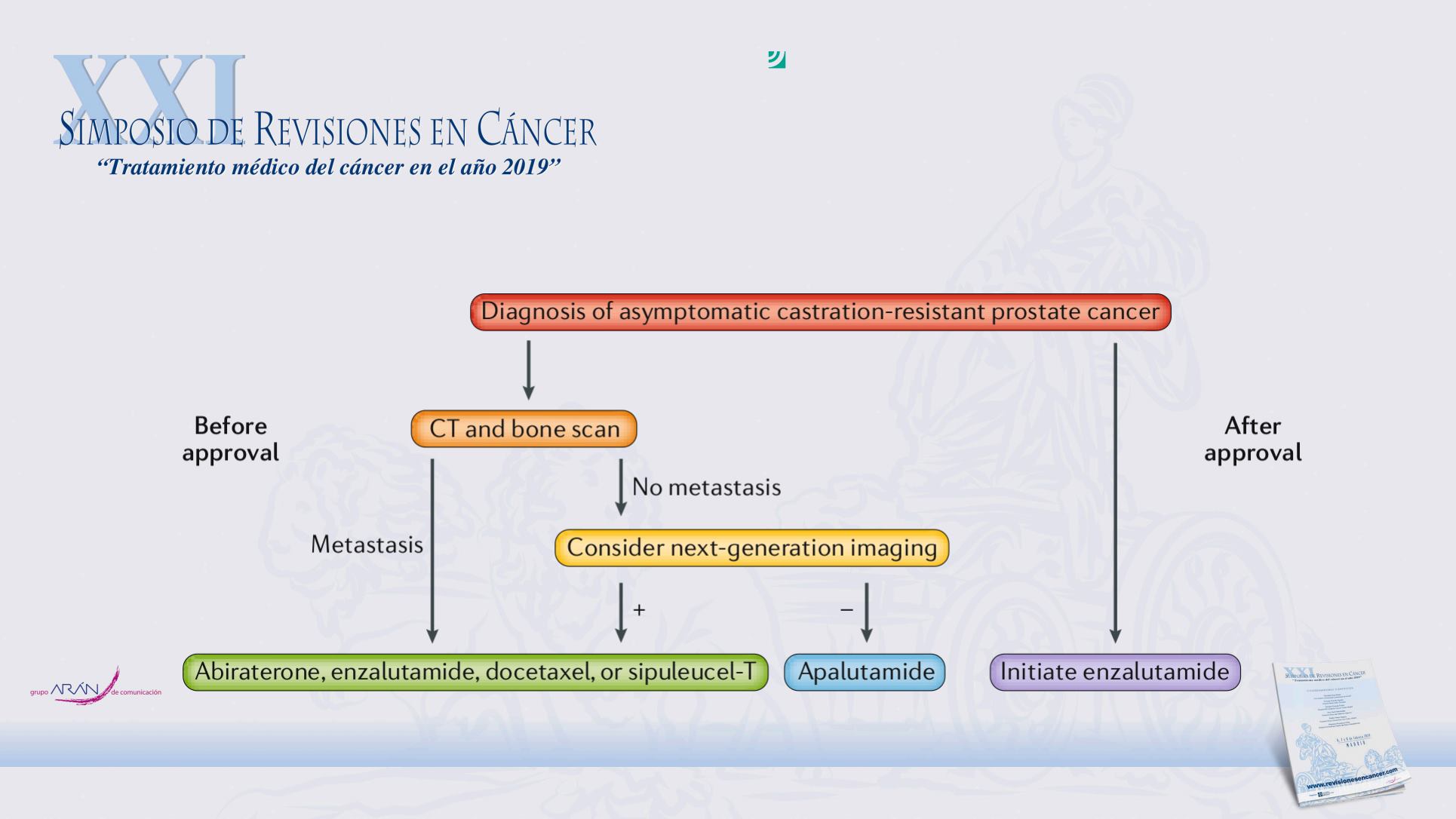
Des
ageme
SPAR
tant un
patient
or whe
detect
those t
referre
or ‘M0
identif
and C
in thes
sugges
ble an
setting
Rec
icine e
tificati
and C
Cance
for nmCRPC on the basis of the SPARTAN
trial, a similar multicentre, double-blind trial
of 1,207 patients
2
. Both SPARTAN and
PROSPER enrolled patients with rising PSA
levels despite castration levels of testoster-
one and no evidence of metastatic disease on
imaging. However, SPARTAN looked only
at conventional CT and bone scan images,
whereas PROSPER also included MRI
results
1
,
2
. Both trials enrolled patients with
similar characteristics, but the PROSPER
participants had shorter PSADTs and a
slightly higher percentage of participants
with a PSADT <6 months than SPARTAN.
Nevertheless, SPARTAN demonstrated a sim-
ilar improvement in MFS with apalutamide
compared with placebo (40.5 months versus
PROS TAT E CANCER
Enzalutamide treatment for
the whole spectrum of CRPC
Hanson Zhao and Stephen Freedland
The FDA recently expanded the indication for enzalutamide for the
treatment of all menwithmetastatic and nonmetastatic castration-resistant
prostate cancer on the basis of the PROSPER trial. Now that both disease
states can have the same treatment, the need to thoroughly identify
metastatic lesions is questioned.
Refers to
Hussain, M. et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer.
N. Engl. J. Med
.
378
, 2465–2474 (2018).
t
enza
basi
Zhao H & Freedland J. Nat Rev Urol 2018;15(11):663-5








