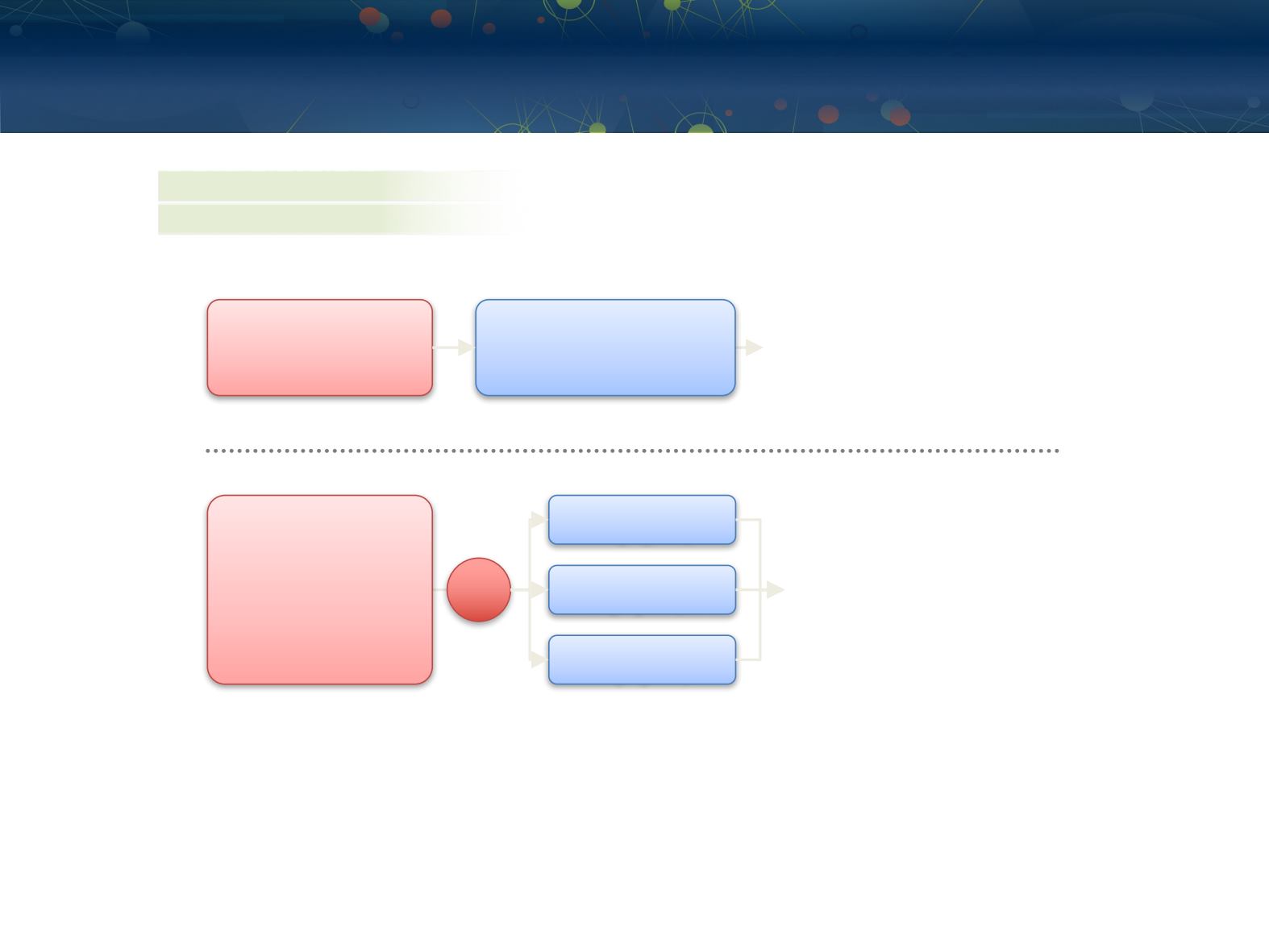
CA209-003 and CA209-010 Study Designs
Phase 1 Study (NCT00730639)
Phase 2 Study (NCT01354431)
Key Inclusion Criteria
• PD after 1–5 systemic
therapies
• ECOG PS ≤2
Nivolumab
1 or 10 mg/kg IV q2w
8-week treatment cycle
•
Treat until confirmed progression
a
or unacceptable toxicity
•
Treatment duration:
–
96 weeks if clinically stable
Endpoints
•
Primary: safety and tolerability
•
Key secondary: ORR
Nivolumab
0.3 mg/kg IV q3w
Nivolumab
2 mg/kg IV q3w
Nivolumab
10 mg/kg IV q3w
Key Inclusion Criteria
• 1–3 prior therapies
• ≥1 prior anti-angiogenic
agent
• PD after last therapy and
≤6 months prior to
enrollment
• KPS ≥70%
•
Treat until confirmed progression
a
or unacceptable toxicity
•
Treatment duration:
–
Continuous if clinically stable
Endpoints
•
Primary: dose response by PFS
•
Key secondary: response rate,
OS, toxicities
a
Treatment beyond progression was permitted if nivolumab was tolerated and clinical benefit was noted.
b
Randomization stratified by MSKCC risk group and number of prior therapies in metastatic setting.
ECOG PS, Eastern Cooperative Oncology Group performance status; IV, intravenous; KPS, Karnofsky performance status; MSKCC, Memorial Sloan Kettering Cancer Center; ORR,
objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; q2w, every 2 weeks; q3w, every 3 weeks; R, randomized.
Included with permission from McDermott DF et al. Oral presentation at ASCO 2016. 4507.
CA209-003: monotherapy
CA209-010: monotherapy
R
b
1:1:1








