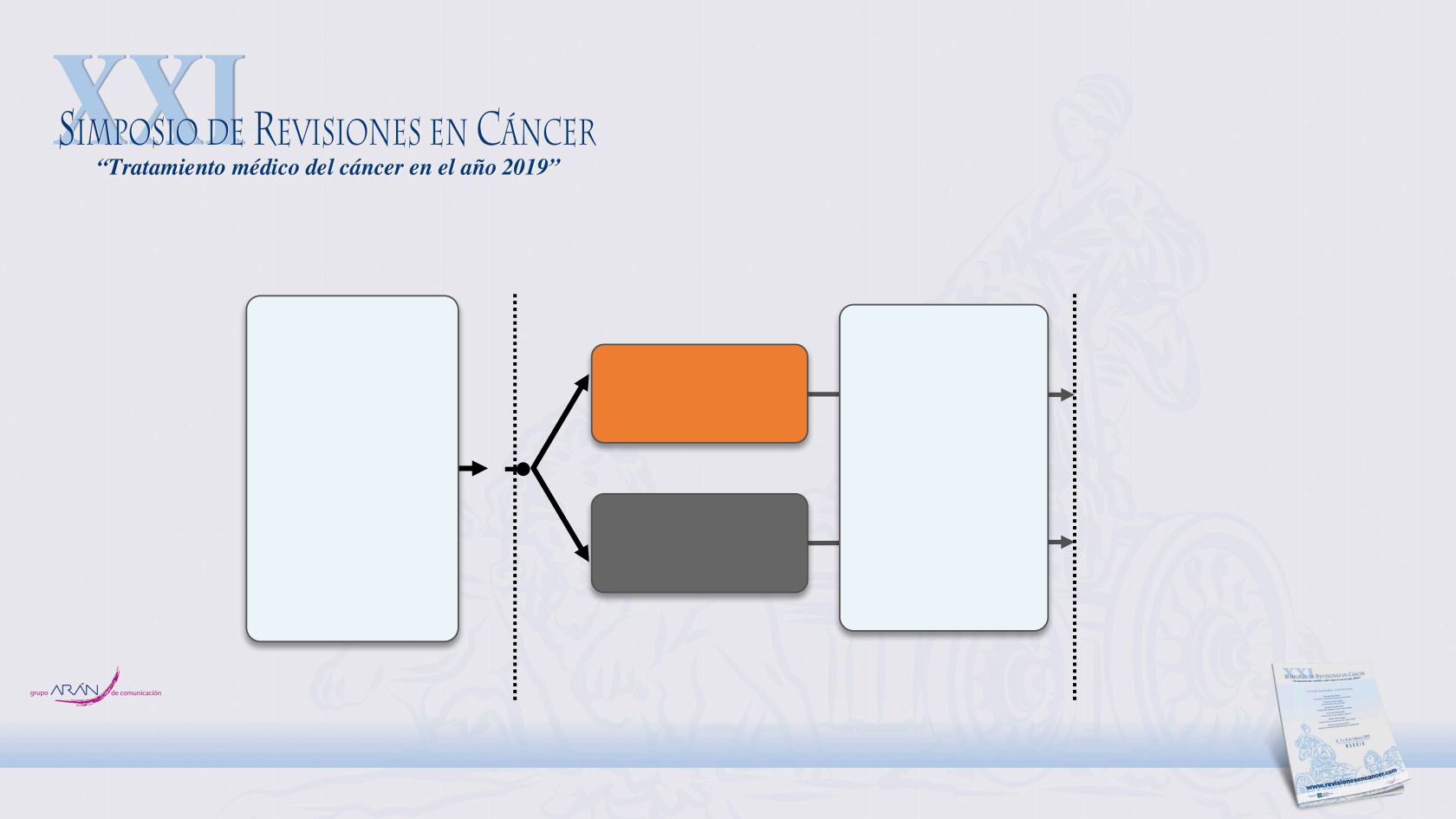
LATITUDE: Phase 3 trial of AA in patients with
newly diagnosed, high-risk, mHNPC
Screening
28 days
Treatment
until disease progression, withdrawal of consent,
or occurrence of unacceptable toxicity
a
Efficacy endpoints
Co-primary:
•
OS
•
rPFS
Secondary: Time to
•
Pain progression
•
PSA progression
•
Next SRE
•
Initiation of
chemotherapy
•
Subsequent therapy
for prostate cancer
Abiraterone (AA) 1000 mg
QD
+ Prednisone 5 mg QD
+ ADT
Placebo 1000 mg QD
+ Placebo 5 mg QD
+ ADT
Randomization 1:1
Patients
•
Newly diagnosed
(≤3 months) adult
men with high-risk
mHNPC
Meets at least 2 of 3
high-risk criteria
•
Gleason score of ≥8
•
Presence of ≥3
lesions on bone scan
•
Presence of
measurable visceral
lesion
Stratification factors
•
Presence of visceral
disease (yes/no)
•
ECOG PS (0, 1 vs 2)
Randomized, double-blind, active-controlled, multicenter phase 3 study
Follow-up
every
4 months
for up to
60 months
Fizazi K et al. N Engl J Med 2017;377(4):352-360 (article and protocol)








