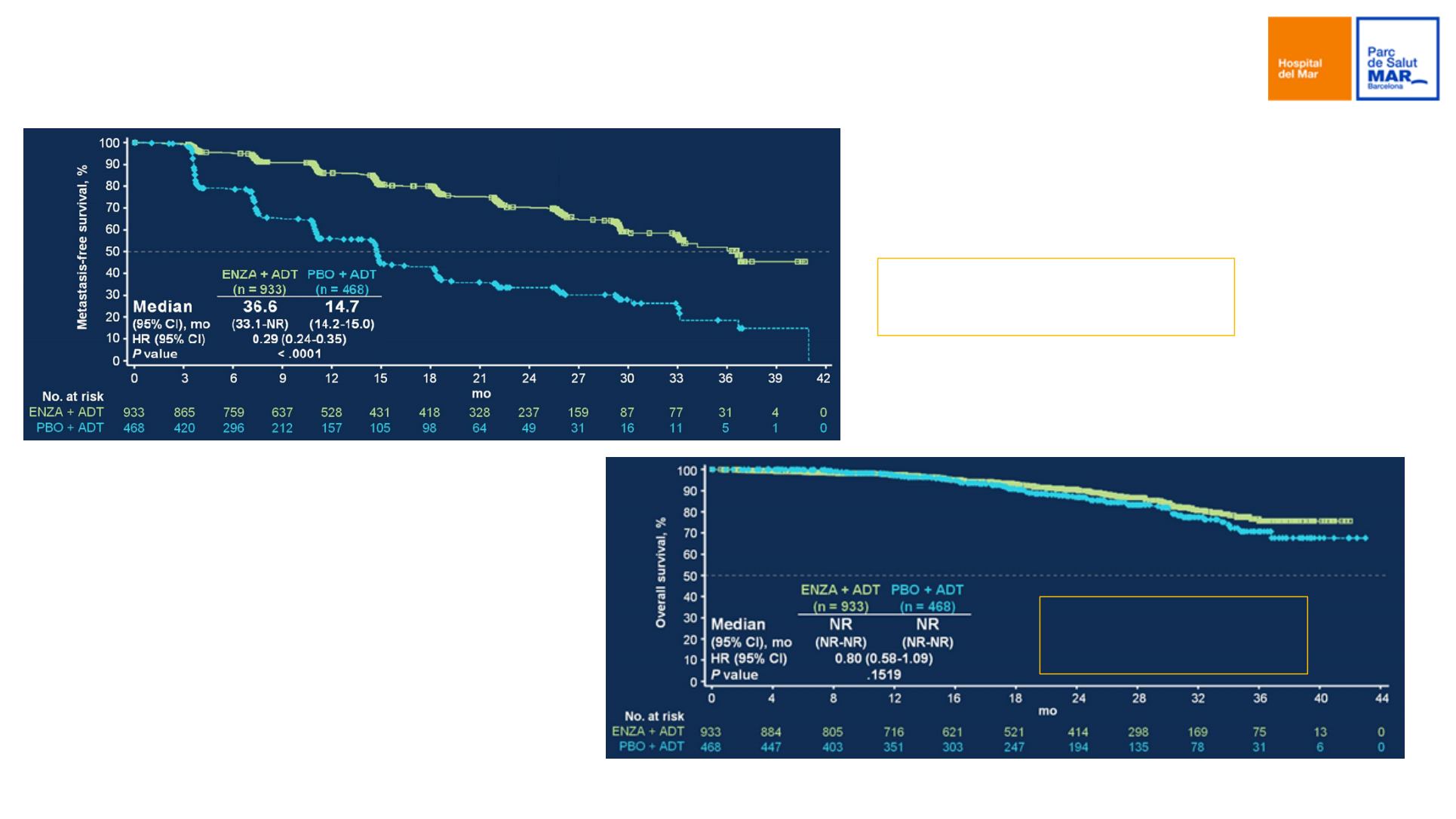
PROSPER phase III trial
Primary endpoint: MFS
71% risk reduction of M1
progression or death
Secondary endpoint: OS
20% reduction risk
of death
Hussain M, et al. PROSPER: A phase 3, randomized, double-blind, placebo-controlled study of enzalutamide in men with nonmetastatic castration-resistant prostate cancer. J Clin Oncol 36, 2018 (suppl 6S;
abstr 3) ASCO GU 2018








