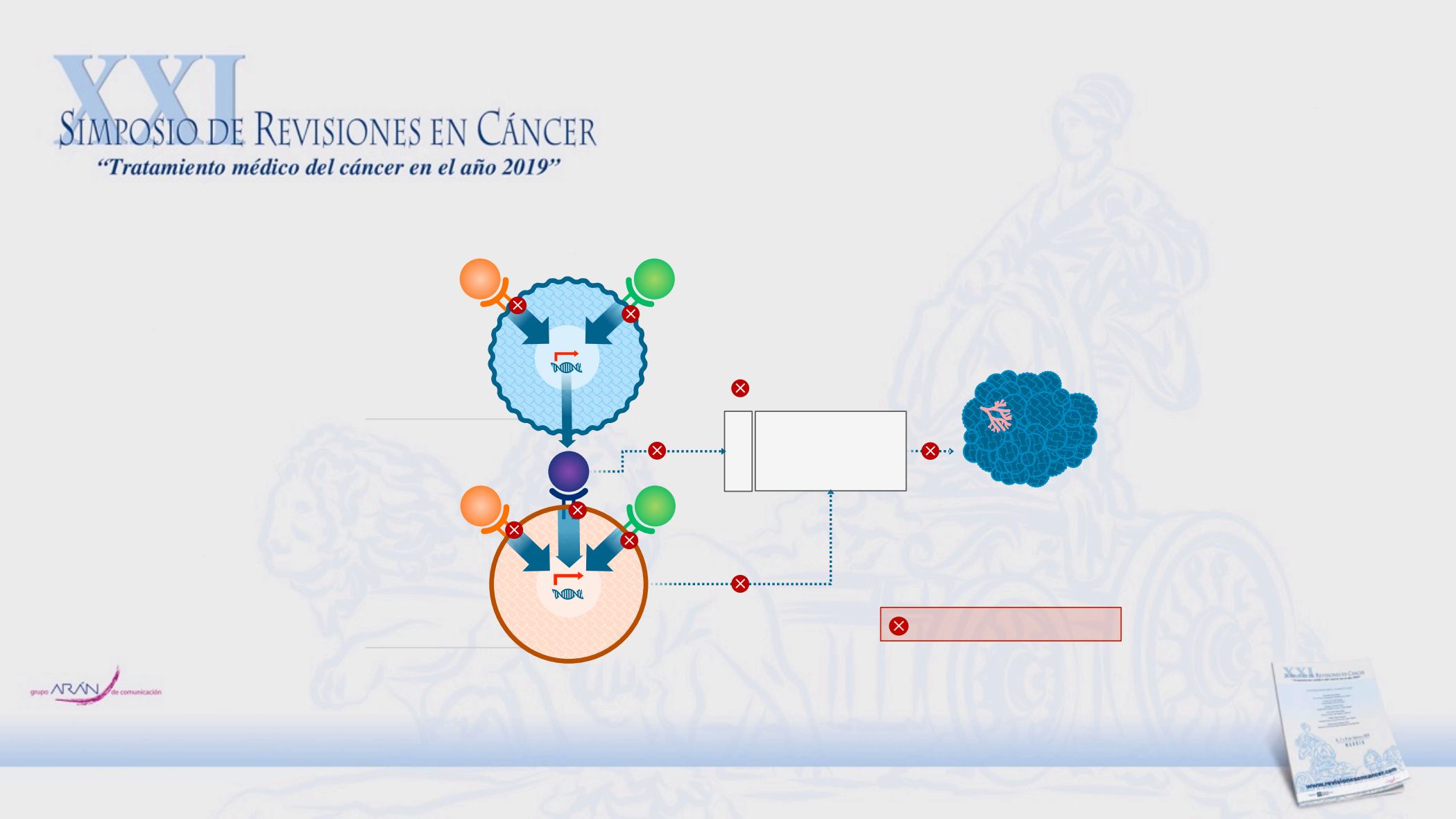
Cabozantinib targets multiple pathways
1-9
1. Goyal L, et al. Clin Cancer Res. 2013;19:2310-2318. 2. Firtina Karagonlar Z, et al. Cancer Sci. 2016;107:407-416. 3. Sennino B, et al. Cancer Discov. 2012;2:270-287.
4. Shojaei F, et al. Cancer Res. 2010;70:10090-10100. 5. Zhou L, et al. Oncogene. 2016;35:2687-2697. 6. Lee HJ, et al. Carcinogenesis. 2014;35:769-775.
7. Carmeliet P, Jain RK. Nature. 2011;473:298-307. 8. Schoenleber SJ, et al. Br J Cancer. 2009;100:1385-1392. 9. Li Y et al. Oncogene 2009;28:3442-3455.
TUMOR CELL
ENDOTHELIAL CELL
Proliferation
Survival
Migration
Invasion
Resistance to VEGFR TKIs
Angiogenesis
AXL
HGF
MET
VEG
F
VEGFR
Tumor Control
Gas6
AXL
HGF
MET
Gas6
Points of cabozantinib inhibition
Cabometyx® (cabozantinib) is approved by the EMA is indicated as monotherapy for the treatment of hepatocellular carcinoma (HCC) in adults who have previously been treated
with sorafenib; pricing and reimbursement is pending in Spain.
1. Goyal L, et al. Clin Cancer Res 2013. 2. Firtina Karagonlar Z, et al. Cancer Sci 2016. 3. Sennino B, et al. Cancer Discov 2012. 4. Shojaei F, et al. Cancer Res 2010. 5. Zhou L, et al. Oncogene 2016.
6. Lee HJ, et al. Carcinogenesis 2014. 7. Carmeliet P, Jain RK. Nature 2011. 8. Schoenleber SJ, et al. Br J Cancer 2009. 9. Li Y et al. Oncogene 2009.








