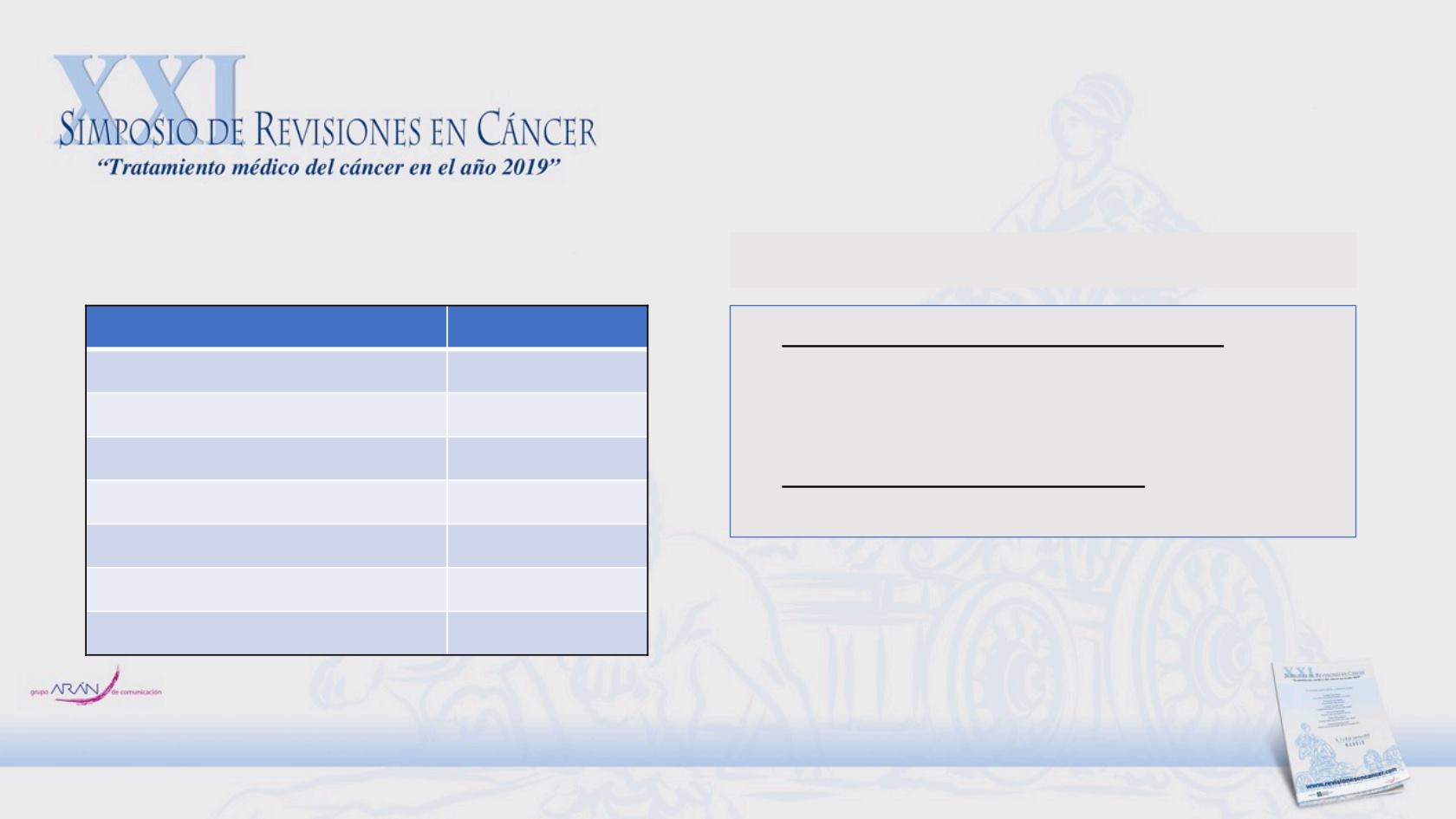
Cabozantinib Phase II study: Efficacy Results
Parameter
Patients, n (%)
RECIST response
Confirmed partial response
2 (5)
Stable disease
31 (76)
Progressive disease
3 (7)
Missing data
4 (10)
Unable to evaluate
1 (2)
Week 12 disease control
27 (66)
•
PFS (months from randomisation)
Cabozantinib 2.5 (95% CI, 1.3–6.8)
Placebo
1.4 (95% CI, 1.3–4.2)
•
OS (months from first dose)
Cabozantinib 11.5 (95% CI, 7.3–15.6)
Secondary end-points
Lead-in stage
Kelley RK., et al. Annals of Oncology 2017;28: 528–534.








