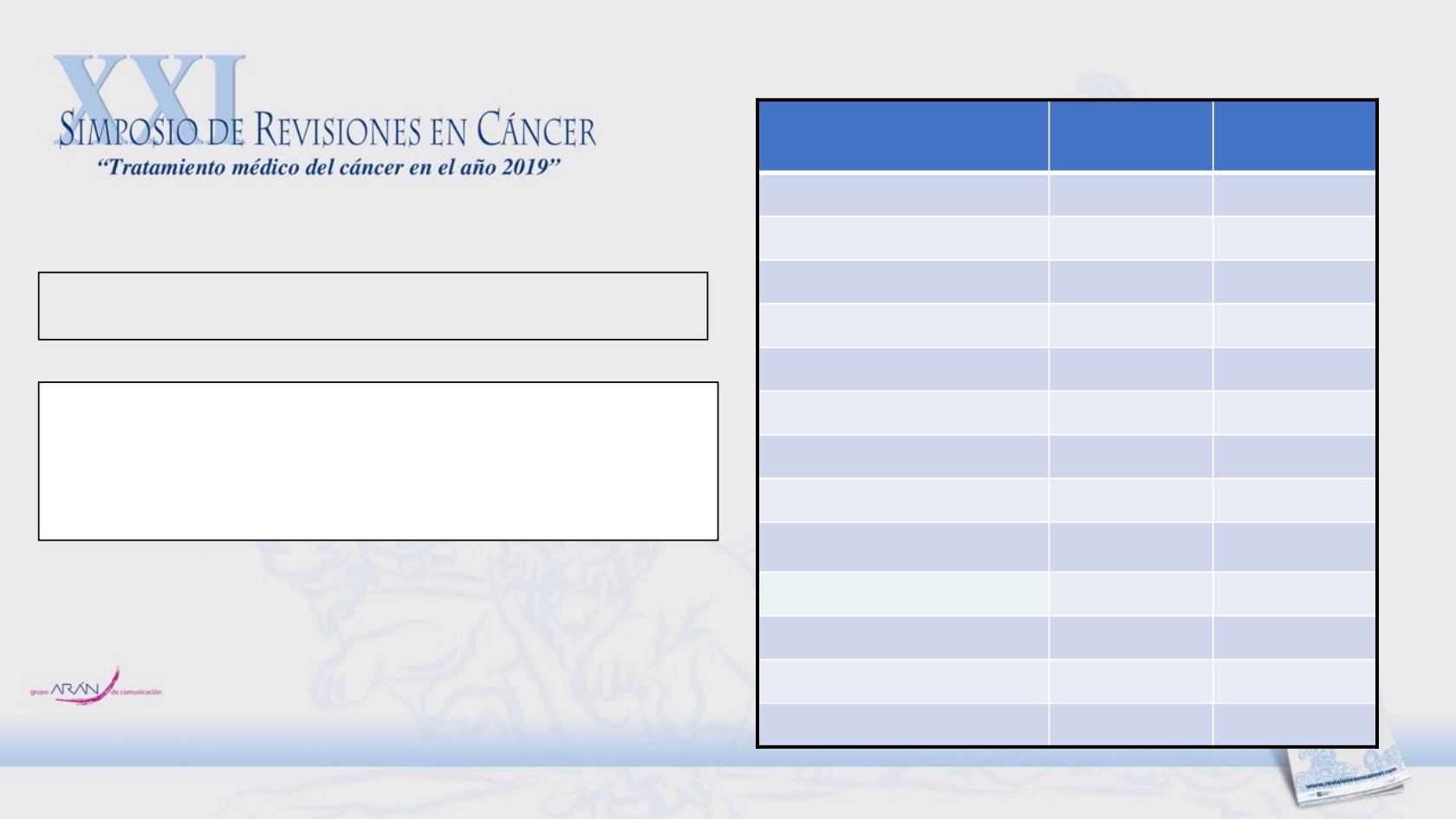
Kelley RK., et al. Annals of Oncology 2017;28: 528–534.
Adverse event
All grades,
n=41 (%)
Grade >3,
n=41 (%)
Any adverse event
41 (100)
35 (85)
Diarrhoea
26 (63)
8 (20)
Hand-foot syndrome
23 (56)
6 (15)
Fatigue
23 (56)
1 (2)
Thrombocytopenia
15 (37)
6 (15)
Nausea
15 (37)
1 (2)
Vomiting
15 (37)
1 (2)
Decreased appetite
12 (29)
0 (0)
Increased AST
11 (27)
4 (10)
Hypertension
10 (24)
4 (10)
Rash
10 (24)
0 (0)
Asthenia
9 (22)
3 (7)
Decreased weight
9 (22)
1 (2)
•
No grade 5 AEs related to the treatment
•
59% of patients had dose reductions
•
Median time to first dose reduction was 39.5 days
Cabozantinib Phase II study: Safety








