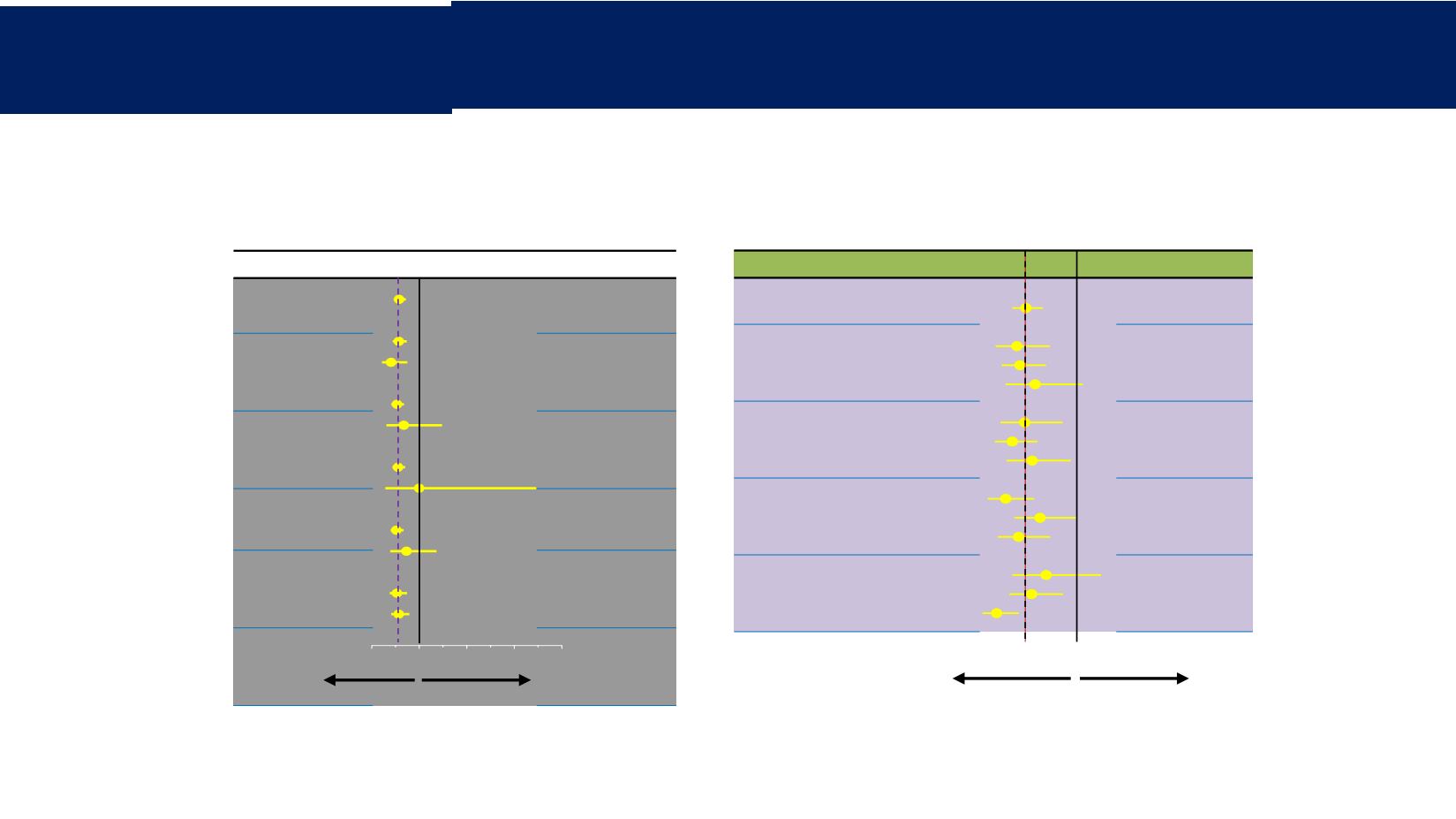
n
HR (95% CI)
All patients
66
6
0.58 (0.46
–
0.72)
ER
+
ER
–
504
62
0.57 (0.44–
0.74)
0.41 (0.22–
0.75)
Rb
+
Rb
–
512
51
0.53 (0.42–
0.68)
0.68 (0.31–
1.48)
Cyclin D1
+
Cyclin D1
–
549
15
0.56 (0.44–
0.71)
1.0 (0.29–3.46)
p16
+
p16
–
466
84
0.52 (0.40–
0.67)
0.73 (0.39–
1.36)
Ki-67
≤20%
Ki-67
>20%
318
235
0.53 (0.38–
0.74)
0.57 (0.41–
0.79)
0
1
2
3
4
HR (95% CI)
Favors PAL+LET
Favors PCB+LET
Percentile n
HR (95% CI)
All patients
666
0.58 (0.46
–
0.72)
ER status
≤25
th
>25
th
to <75
th
≥75
th
142
282
142
0.50 (0.32–0.78)
0.53 (0.37–0.74)
0.65 (0.41–1.05)
Rb status
≤25
th
>25
th
to <75
th
≥75
th
154
249
160
0.57 (0.36–0.88)
0.46 (0.32–0.67)
0.63 (0.42–0.95)
Cyclin D1
status
≤25
th
>25
th
to <75
th
≥75
th
141
247
176
0.41 (0.26–0.65)
0.69 (0.48–1.00)
0.52 (0.34–0.78)
p16 status
≤25
th
>25
th
to <75
th
≥75
th
140
258
152
0.74 (0.46–1.20)
0.62 (0.44–0.89)
0.33 (0.21–0.52)
0.0
0.5
1.0
1.5
HR (95% CI)
Favors PAL+LET
Favors PCB+LET
Análisis cualitativo
Análisis cuantittivo
Finn RS, et al.
Ann Oncol
2016;27(Suppl. 6) (Abstract LBA15)
IC, intervalo de confianza; RE, receptor de estrógeno; HR, cociente de riesgo; LET, letrozol;
PAL, palbociclib; PCB, placebo; Rb, retinoblastoma.
moleculares a asociar al RE/RPg
Palbociclib
CMM RE[+]








