San Antonio Breast Cancer Symposium, December 6 - 10, 2016
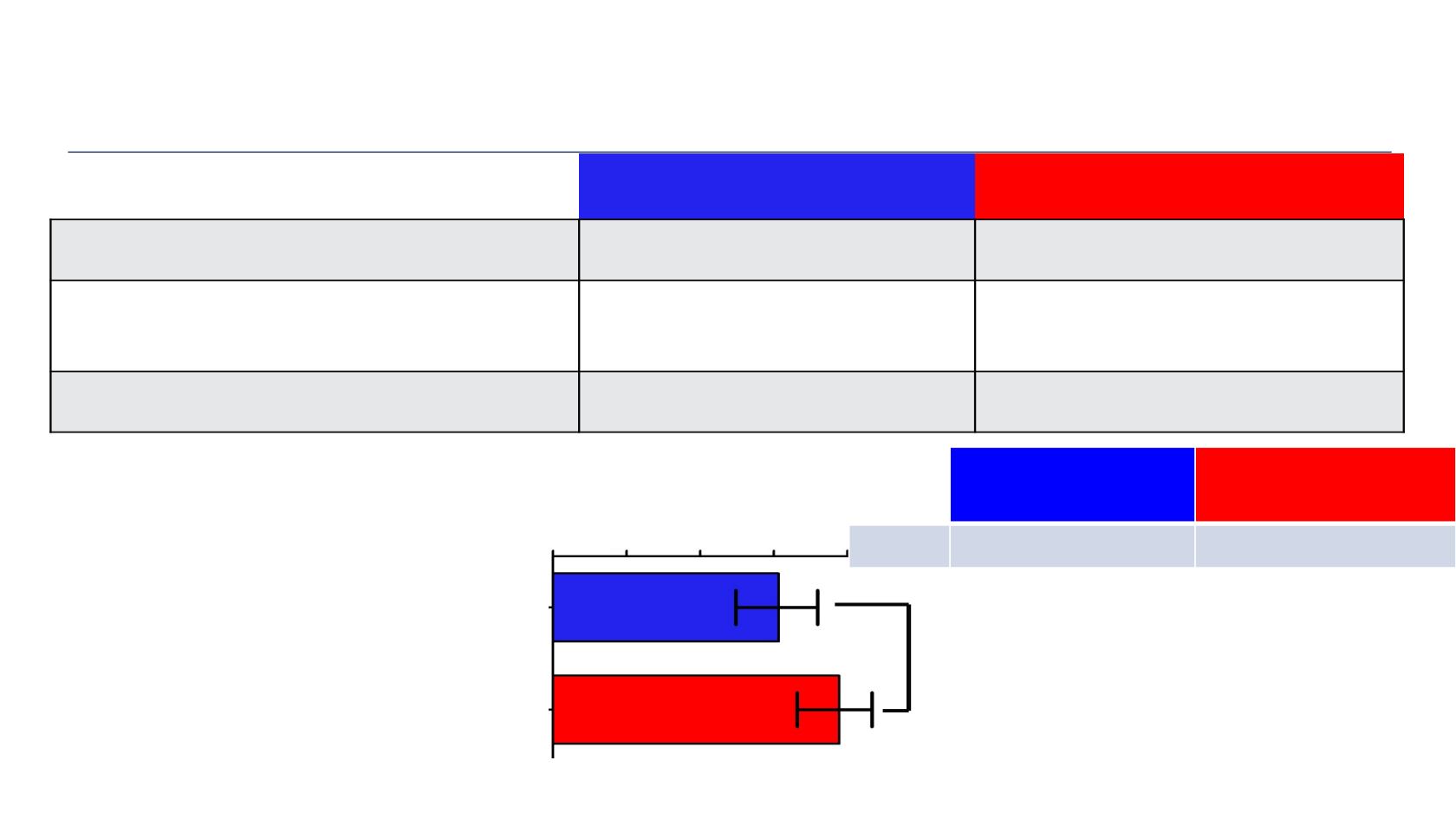
Tumor Response
Placebo + C/P
N = 98
Veliparib + C/P
N = 95
ORR (CR + PR),
n/N, % (95% CI)
49/80 (61.3%)
(49.7–71.9)
56/72 (77.8%)*
(66.4–86.7)
CBR
(week 18 progression-ĨƌĞĞ ƌĂƚĞͿ͕
% (95% CI)
87.0%
(78.3–92.4)
90.7%
(82.2–95.2)
DOR,
median months, (95% CI)
11.1
(9.5–15.7)
11.7
(8.5–14.1)
WƌŽƉŽƌƚŝŽŶ ŽĨ WĂƚŝĞŶƚƐ ǁŝƚŚ
ORR, %
(95% CI)
49/80
(61.3%)
56/72
(77.8%)
Placebo
+ C/P
Veliparib
+ C/P
0
20 40 60
80 100
P = 0.027
*P <0.05 for placebo +C/P vs veliparib + C/P.
Tumor assessments were per independent radiology reviewer. ORR, CR, and PR shown represent confirmed responses; these analyses included all patients with
measurable disease at baseline. DOR analysis included all patients with an objective response. CBR analysis included all randomized patients who had a
deleterious BRCA1/2 mutation per the core lab.
Placebo + C/P
N = 98
Veliparib + C/P
N = 95
ORR
61.3%
77.8%
Han H. SABCS 2016








