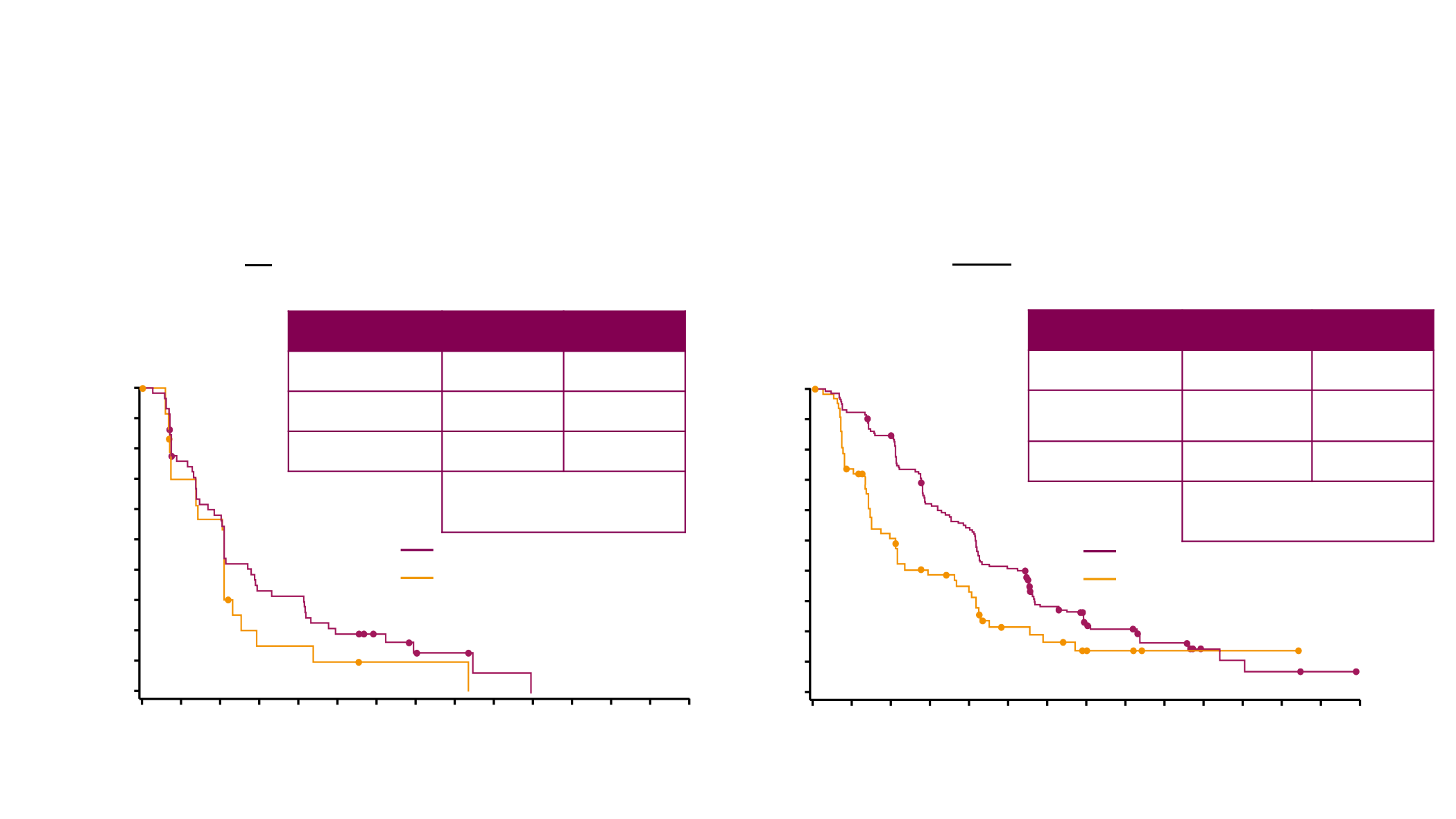
Olaparib does not seem to improve PFS compared with
TPC in patients who had received prior platinum
Patients who had received prior platinum for breast cancer
Patients who had not receive prior platinum for breast cancer
11
2
18
3
33
13
35
13
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0.0
Probability of progression-free survival
Time from randomisation (months)
14
12
10
8 6 4 2 0
16 18 20 22 24 26 28
3
1
7
1
11
2
18
3
19
3
43
16
60
26
3
1
1
0
0
0
0
0
0
0
0
0
0
0
3
1
6
1
13
2
24
5
58
24
1
0
1
0
0
0
0
0
0
0
0
0
Olaparib
Chemotherapy
Number of patient’s at risk
Olaparib 300 mg bd (N=60)
Chemotherapy (N=26)
105
24
50
9
82
21
121
31
124
33
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0.0
Probability of progression-free survival
Time from randomisation (months)
14
12
10
8 6 4 2 0
16 18 20 22 24 26 28
20
3
33
7
58
9
76
18
88
22
134
47
145
71
18
3
10
1
4
1
3
1
2
1
1
0
0
0
18
3
30
6
60
11
143
64
10
1
10
1
3
1
2
1
1
0
1
0
Olaparib
Chemotherapy
Number of patient’s at risk
Olaparib 300 mg bd (N=145)
Chemotherapy (N=71)
Olaparib TPC
n
145
71
Events (%)
113 (77.9)
50 (70.4)
Median (m)
8.3
4.2
HR= 0.60
95% CI (0.43, 0.84)
Olaparib TPC
n
60
26
Events (%)
50 (83.3)
21 (80.8)
Median (m)
4.2
4.2
HR= 0.67
95% CI (0.41, 1.14)
Robson ME. NEJM 2017








