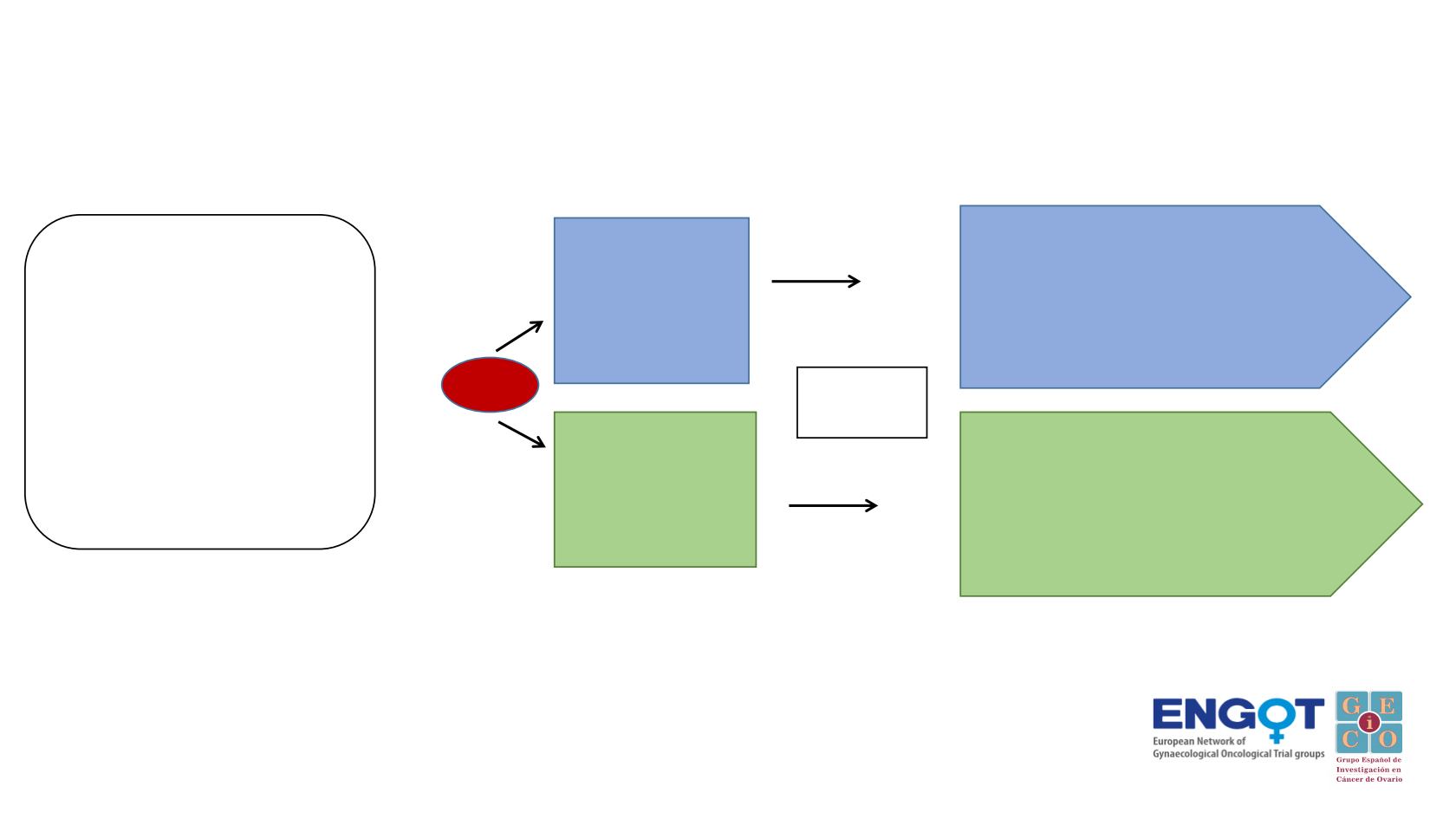
•
Recurrent high- grade
serous or endometrioid, or
undifferentiated ovarian,
primary peritoneal or tubal
carcinoma
•
TFIp >6 months
•
≤ 2 prior lines
•
Measurable disease
•
ECOG≤ 1
Stratification factors
:
•
Platinum based regimen selected
•
PFI (6-12 months vs > 12 months)
•
BRCA mutation status (mutated
vs. non-mutated)
Primary Endpoint:
•
PFS by RECIST v.1.1
Secondary endpoints:
•
Safety and tolerability
•
TFST, TSST,PFS2,OS
•
ORR, DOR
•
QoL/PRO
1:1
RANDOMIZATION
Platinum
doublet +
Placebo
6 cycles
Platinum-
doublet +
Atezolizumab
6 cycles
RECIST v1.1 CT SCAN
If CR, PR
or SD
Niraparib + Placebo
until disease progression
Niraparib + Atezolizumab
until disease progression
A
B
N= 414 patients
Combination of PARPi and immunotherapy
ANITA study: TFIp > 6 months
PI: Dr González-Martín








