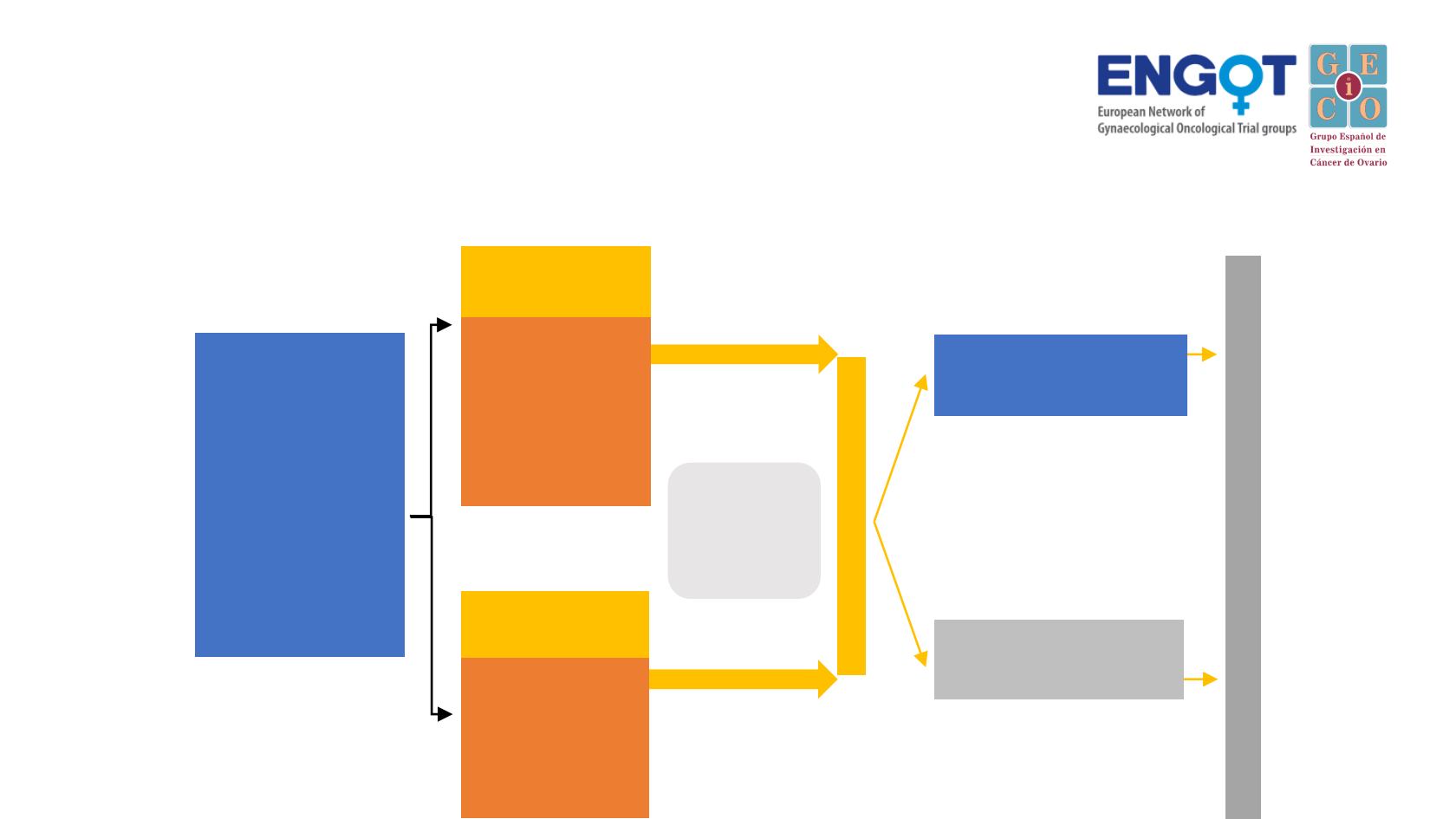
PARPi after PARPi
OReO – ENGOT-Ov38
(
O
laparib
Re
treatment in late recurrent
O
varian cancer)
R
A
N
D
O
M
I
Z
A
T
I
O
N
Eligible patients
§
Relapsed non-
mucinous EOC
§
Documented
BRCA1/2
status
§
Treatment with one
course of PARPi
maintenance
therapy
§
PR/CR after≥4
cycles of platinum-
based chemo
Olaparib tablets
300 mg bid or
last tolerable dose
Placebo
PFS
Primary endpoint
(RECIST 1.1)
PFS, OS, TTP
‡
, TDT, TFST, TSST, HRQoL, Safety
Stratification factors:
•
Prior bevacizumab
•
≤3 vs ≥4 prior lines of
chemotherapy
136 patients
with a germline or somatic
mutation in
BCRA
1/2
Exposure for ≥18 months
after first-line Cx or ≥12
months after second-/later-
line chemotherapy
Cohort 1
BRCA
m
280 patients
Exposure for ≥12 months
after first-line Cx or ≥6
months after second-/later-
line chemotherap
y
Cohort 2
non-
BRCA
m
PR or CR
to
most recent
course of
platinum-based
chemotherapy
(no bevacizumab)








