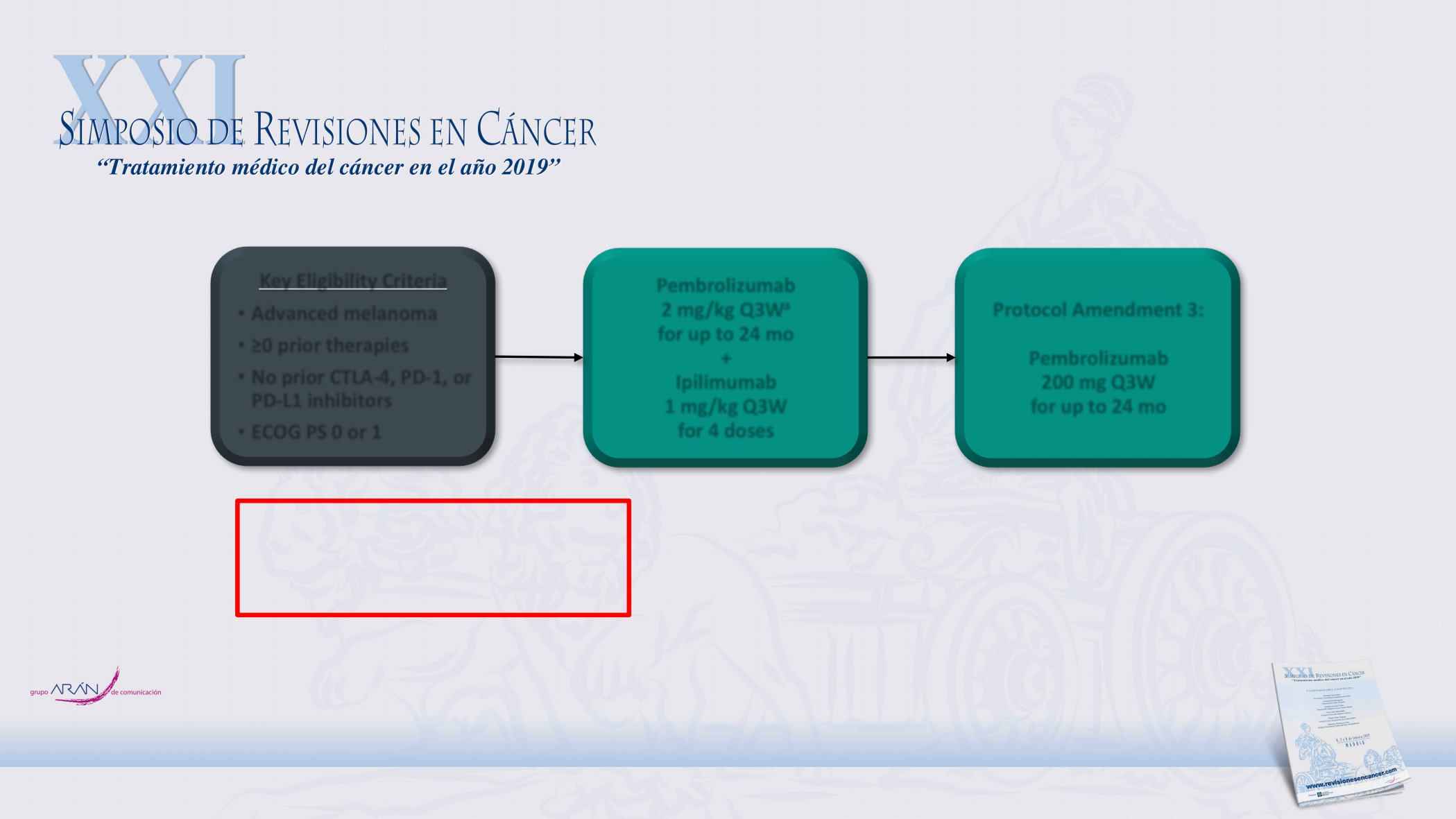
KEYNOTE-029 Cohort 1B
ClinicalTrials.gov identifier, NCT02089685.
LONG SMR 2018
Key Eligibility Criteria
•
Advanced melanoma
•
≥0 prior therapies
•
No prior CTLA-4, PD-1, or
PD-L1 inhibitors
•
ECOG PS 0 or 1
Pembrolizumab
2 mg/kg Q3W
a
for up to 24 mo
+
Ipilimumab
1 mg/kg Q3W
for 4 doses
Protocol Amendment 3:
Pembrolizumab
200 mg Q3W
for up to 24 mo
•
Primary end point:
safety
•
Secondary end points:
OR, DOR, PFS,
OS
Analysis at 17-mo median follow-up
1
•
45% with ≥1 grade 3-4 treatment-related AE
•
61% ORR, 15% CR
•
69% 1-year PFS rate
•
89% 1-year OS rate
1. Long GV et al.
Lancet Oncol
2017;18:1202-10.








