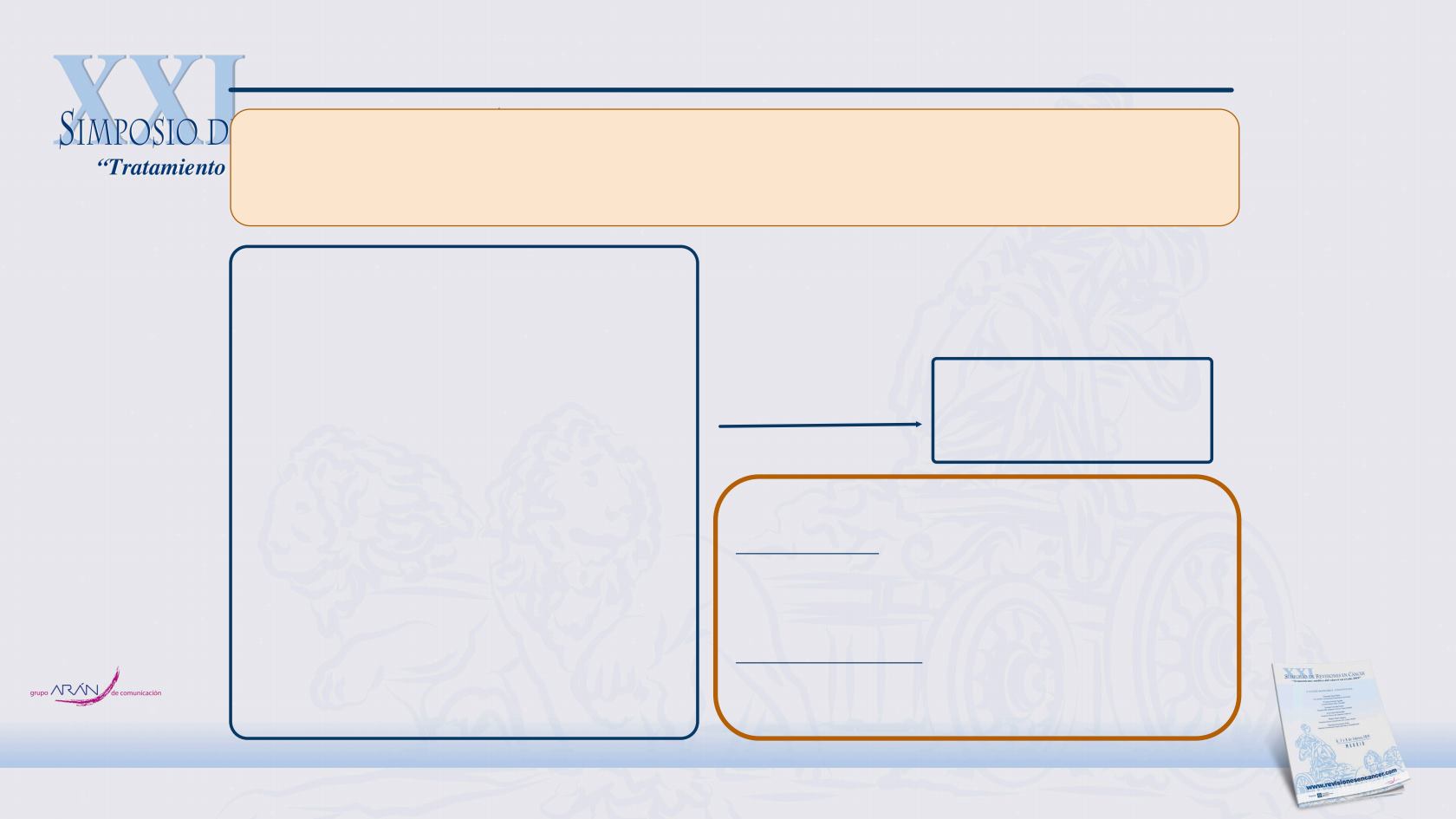
MAIN EXCLUSION CRITERIA:
Prior
systemic
treatment
for
MUM.
Prior treatment with any drug specifically targeting T-
cell costimulation.
Active CNS metastases.
NIVO (1 mg/kg, iv, q3
weeks) and 4 doses of IPI (3
mg/kg iv q3 weeks) followed
by NIVO (3 mg/kg q2 weeks)
STUDY DESIGN
MAIN
INCLUSION CRITERIA:
Histologically confirmed MUM not suitable for radical
treatment, age ≥18 years old, ECOG 0-1, measurable
disease by CT or MRI as per RECIST 1.1. Adequate
haematologic/organ function.
●
P
rogression disease
●
T
oxicity
●
Consent W
ithdrawal
Primary endpoint: To determine the efficacy, in terms of overall
survival (OS) at 12 months, after starting treatment with
nivolumab combined with ipilimumab in patients with
metastatic
uveal
melanoma.
Secondary endpoints:
Evaluate the safety profile,
Progression-Free Survival (PFS), Objective Response Rate (ORR)
(According
the
RECIST
criteria
1.1).
Phase 2 study of nivolumab
combined with ipilimumab in
subjects with previously
untreated metastatic uveal
melanoma.
ENDPOINTS
BACKGROUND
Uveal melanoma (UM) accounts for 0.1% of cancer-related deaths. Liver disease is the most common finding. Life expectancy is
reduced (<9 months) and chemotherapy seems not to improve overall survival (OS). Nivolumab plus ipilimumab has showed
efficacy in metastatic skin melanoma, but to date no evidence on metastatic UM (MUM) is available.
JM Piulats
et al.
ESMO 2017








