RESULTS (2)
Responses by RECIST
v1.
1
(N=
5
2)
N
% (CI95%)
Complete Responses (CR)
0
0
Partial Responses (PR)
6
11.5 (2.9;20.2)
Stable Disease (SD)
24 46.2 (32.6-59.7)
Progressive Disease (PD)
22
42.3(28.9-55.7)
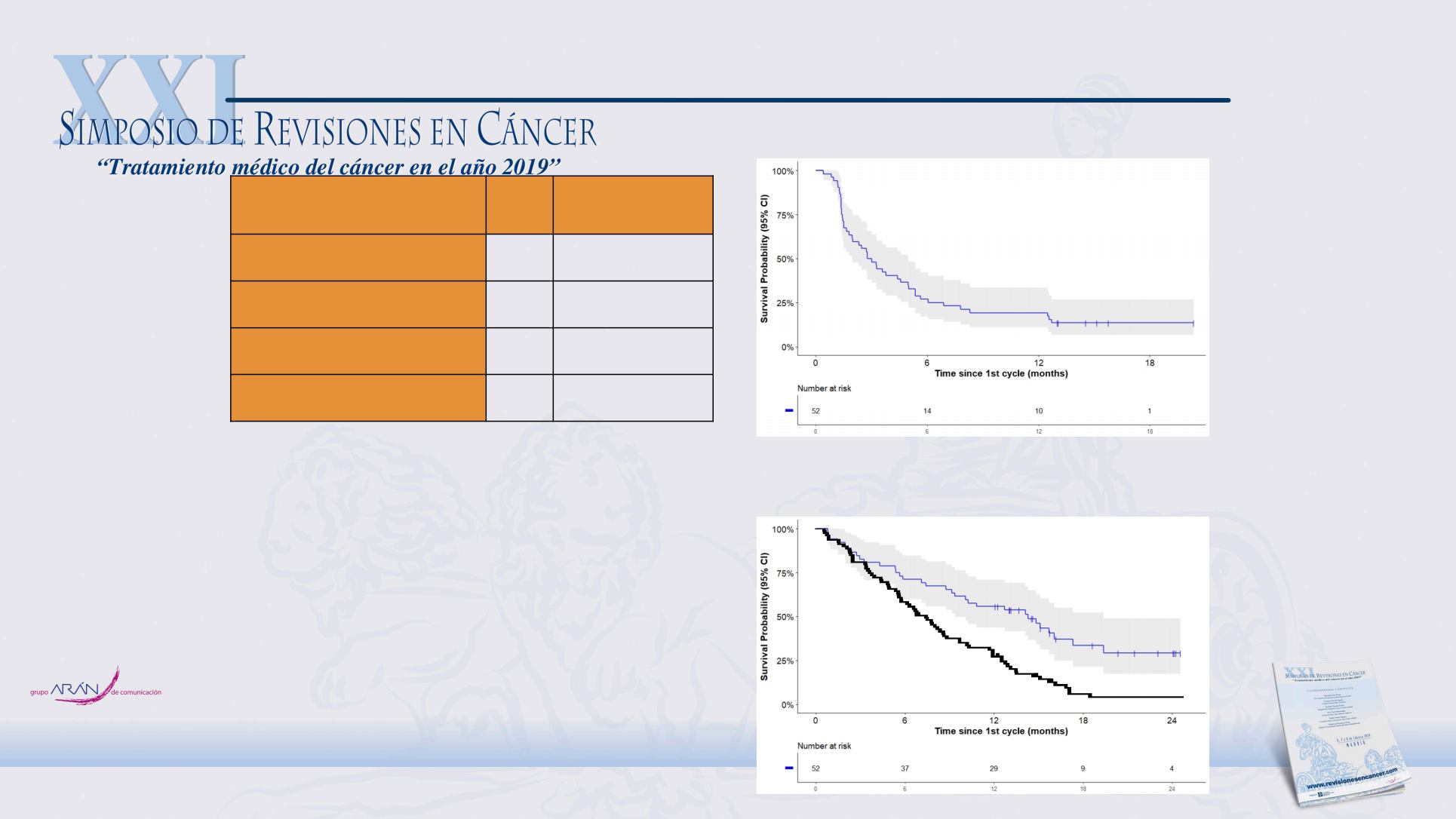
Overall survival at 12 months is 55.8%
(95%CI: 42.3-69.3) with a median follow-
up time of 12.86 months.
Combination of nivolumab + ipilimumab
is feasible in terms of efficacy and
toxicity for MUM that merit further
investigations.
Table 3. Objective Response Rate
Figure 1. Progression Free Survival
Figure 2. Overall Survival
Median PFS: 2.78 months
(95%CI: 1.80-3.75)
Median OS: 14.28 months
(95%CI: 9.44-19.12)
JM Piulats
et al.
ESMO 2017








