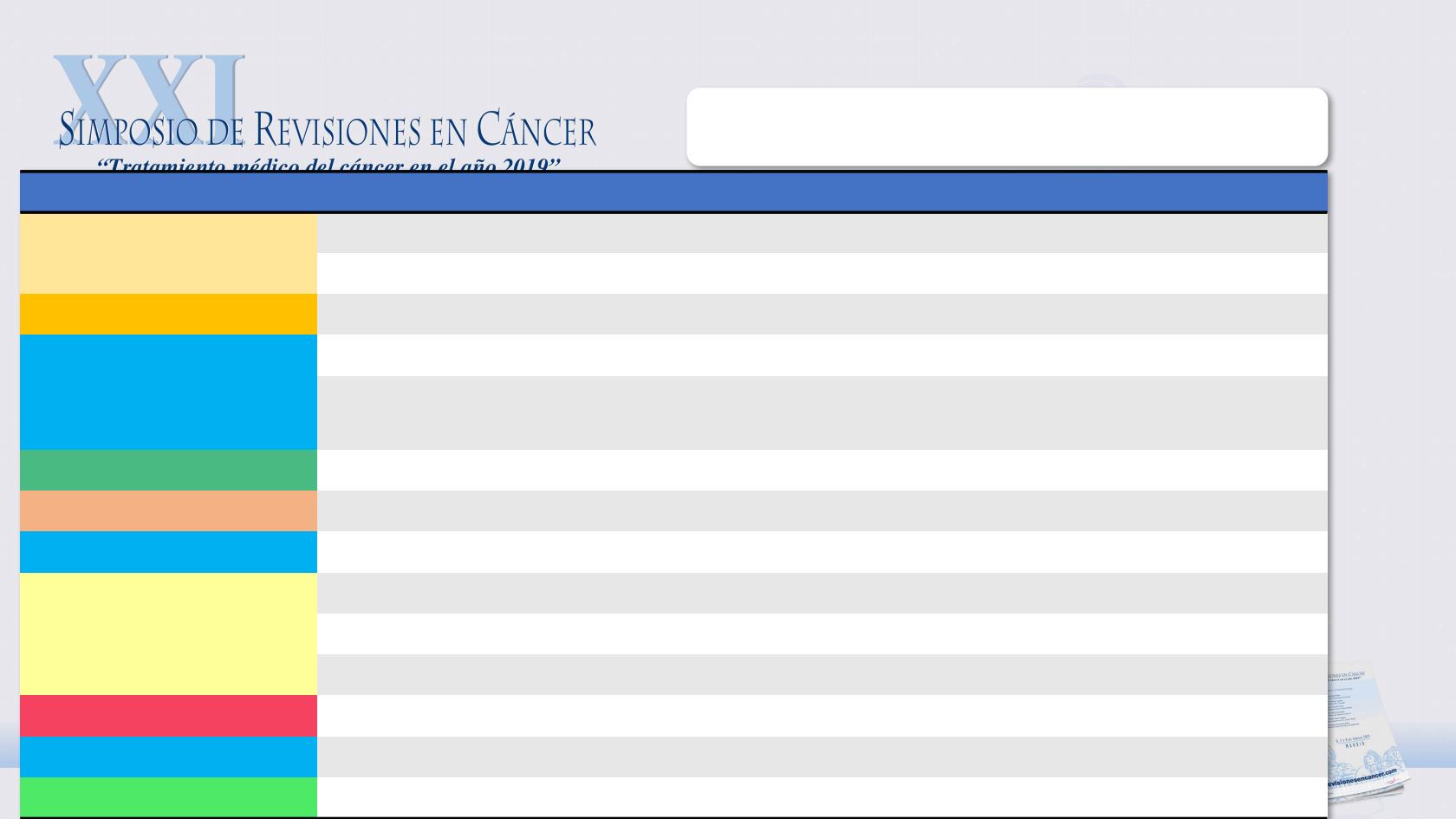
CURRENTLY ONGOING CLINICAL TRIALS WITH NEW
TREATMENT STRATEGIES
STUDY
DRUGS
ENDPOINT
NCT IDENTIFIER
OCCLURANDOM
Lutathera vs sunitinib
12m-PFS
NCT02230176
COMPETE
Lutathera vs Everolimus
PFS
NCT03049189
AXINET
Somatostatin Analog + Axitinib/Placebo
PFS
NCT01744249
CABINET
Cabozantinib vs Placebo after Everolimus
PFS
NCT03375320
SEQTOR
Everolimus
è
STZ/5FU vs
STZ/5FU
è
Everolimus
Second PFS
NCT02246127
CLARINET FORTE
High dose Lanreotide
PFS
NCT02651987
S543/310
Everolimus
Biomarkers
NCT02305810
RESUNET
Sunitinib rechallenge
6m-PFS
NCT02713763
DUNE
Durvalumab + Tremelimumab
ORR
NCT03095274
CPDR001E2201
PDR001
ORR
NCT02955069
PLANET
Pembrolizumab + Lanreotide Depot
ORR
NCT03043664
CONTROL NETS
TEMCAP vs PRRT vs TEMCAP +PRRT
PFS
NCT02358356
MCC-18141
Ibrutinib
ORR
NCT02575300
GETNE1408 - SUNEVO
Sunitinib + Evofosfamide
ORR
NCT02402062








