EVEROLIMUS
TARGETED AGENTS
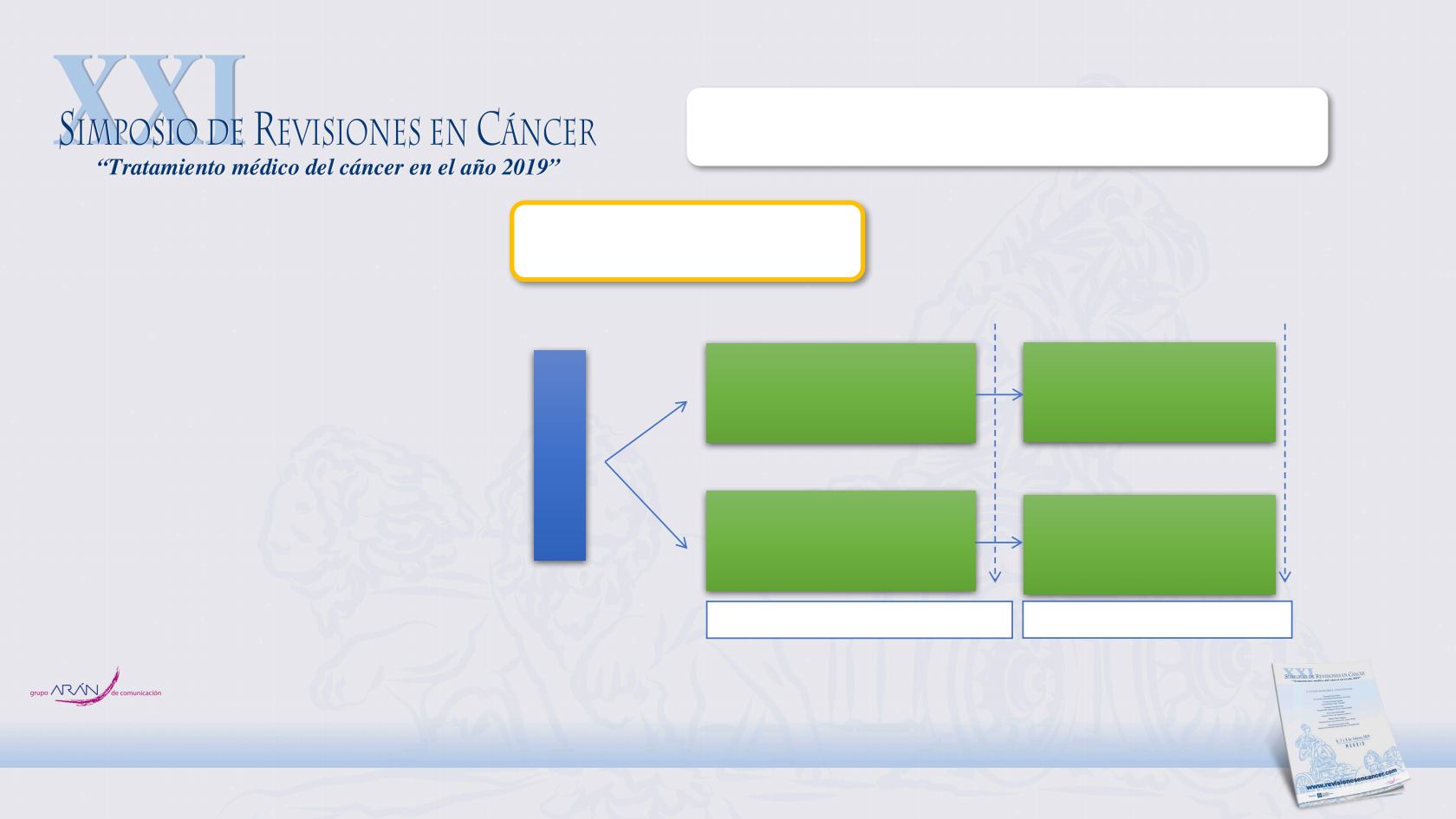
Yao JC, et al. N Engl J Med 2011; 364:514-523
Advanced pNET (N=410)
- Adv well/mod differentiated
- Radiologic progression ≤ 12m
- Prior antitumor therapy allowed
- WHO PS ≤ 2
Primary endpoint: PFS
RANDOMISE
Everolimus 10 mg/day
+ BSC
(N=207)
Placebo
+ BSC
(N=203)
1:1
Open-Label extension phase
Everolimus 10 mg/day
+ BSC
(N=53)
Everolimus 10 mg/day
+ BSC
(N=172)
Core phase (double-blind phase)
Primary analysis
Final OS analysis
RADIANT-3








