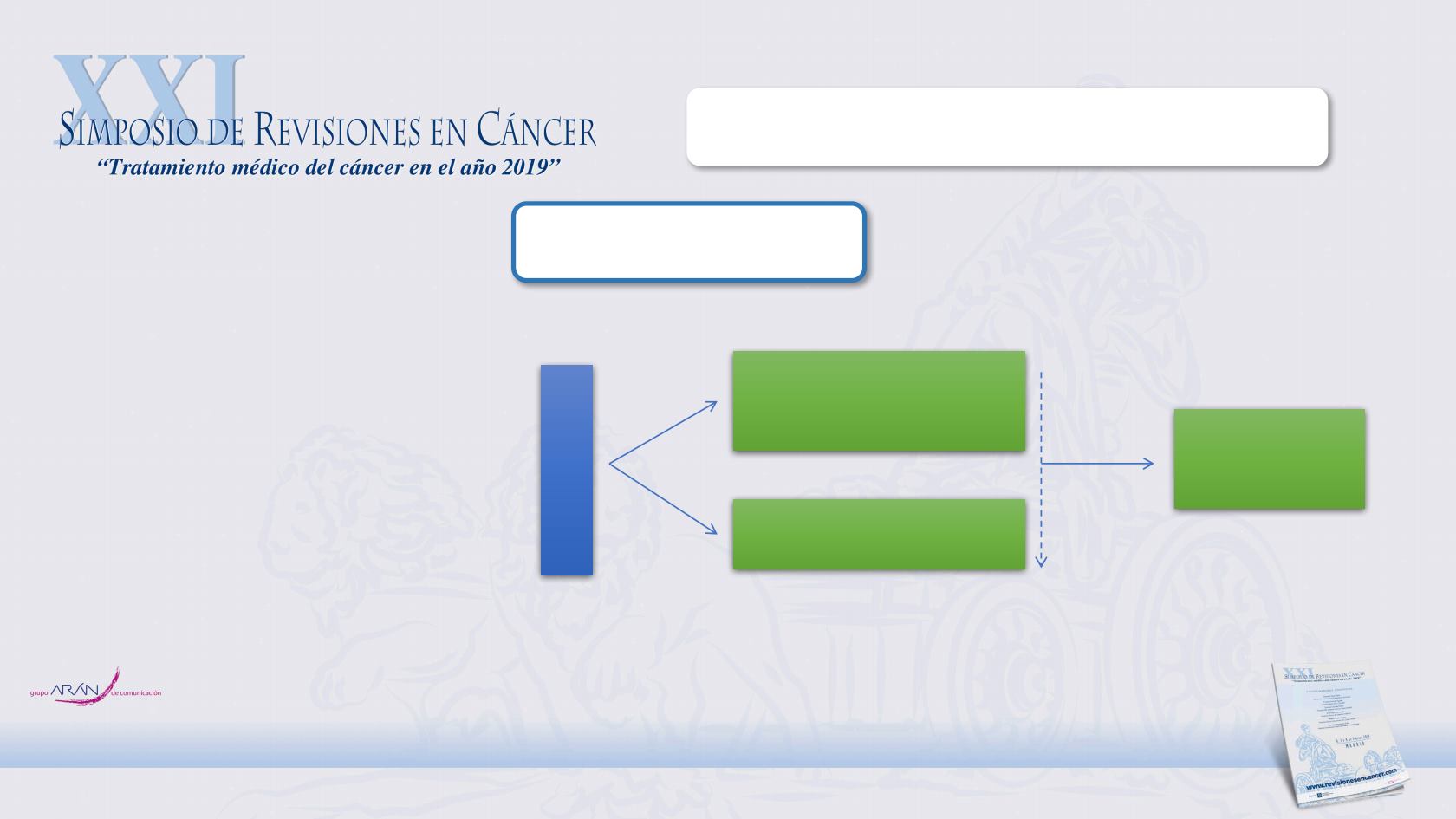
TARGETED AGENTS
SUNITINIB
Advanced pNET (N=171/340 planned)
- Well differentiated
- Radiologic progression ≤ 12m
- Prior antitumor therapy allowed
(except TKI/VEGFR)
- WHO PS ≤ 1
Primary endpoint: PFS
RANDOMISE
Sunitinib 37.5 mg/day
continuous daily dosing
(N=86)
Placebo
(N=85)
1:1
Raymond E, et al. N Engl J Med 2011;364:501-13.
Faivre S, et al. Ann Oncol 2017; 28:339-343
Primary analysis
(data and safety monitoring committee)
Trial
closure
Open-label
sunitinib on
extension study








