EMA Guideline on similar biological medicinal products containing Biotechnology-derived proteins as active substance: quality issues.
EMEA/CHMP/BWP/49348/2005
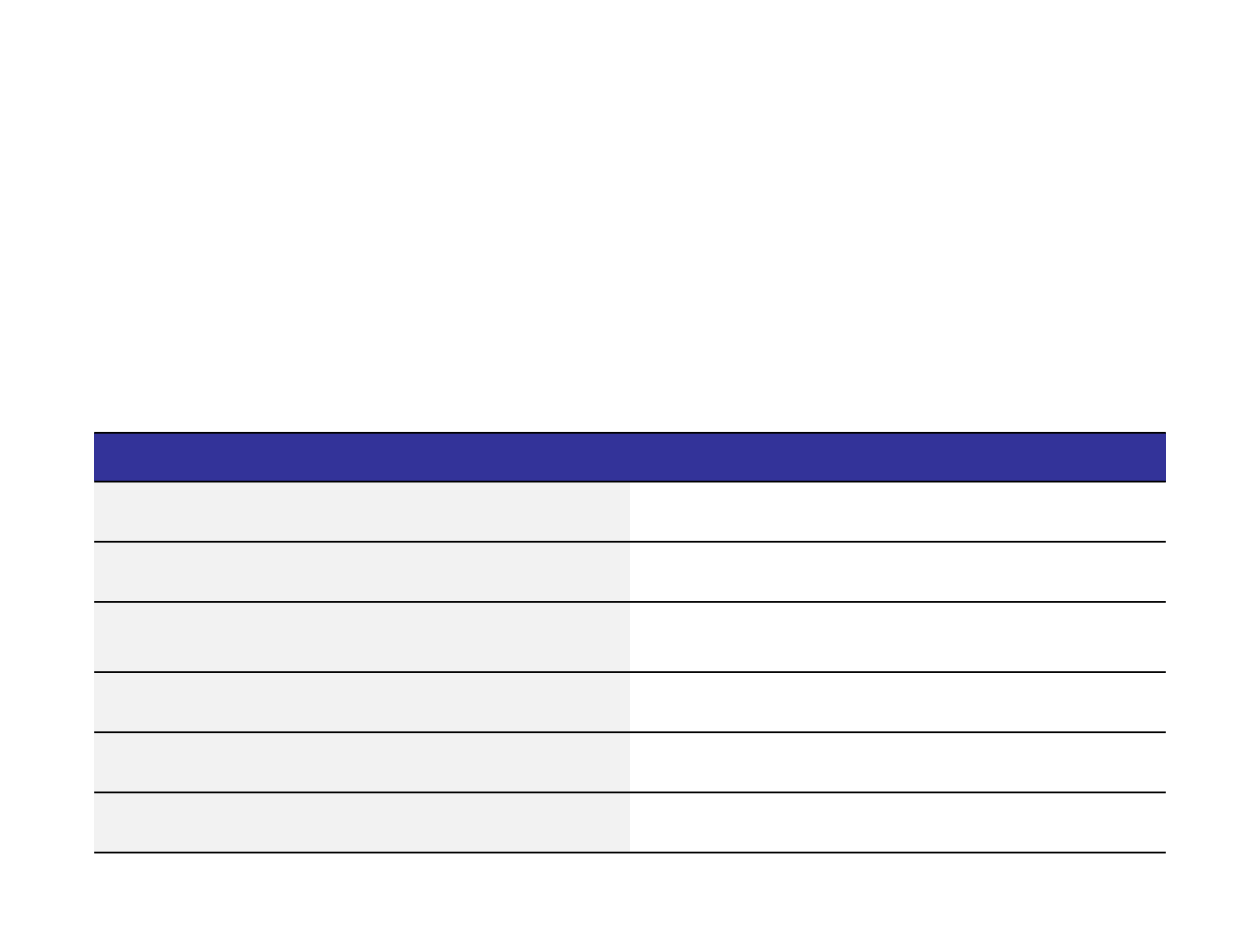
Characteristic Analyzed
Analytical Method
Primary structure
Peptide mapping
Higher order
Disulphide bond analysis
Content
Protein concentration
Purity (aggregates and fragments)
Analytical ultracentrifugation
Isoforms (charge variants)
Ion-exchange chromatography
Glycosylation
Oligosaccharide profile
Step 1: Physico-Chemical
characterization








