•
Checkmate 568 is a phase 2 study of
nivolumab (3 mg/kg Q2W) + ipilimumab (1
mg/kg Q6W) in 1L advanced NSCLC
1
•
Primary endpoint of ORR in PD-L1 ≥1% and 1%
populations
1
•
Retrospective testing from Checkmate 026,
012, and 568 informed selection of the TMB
cutoff (≥10 mut/Mb)
1-5
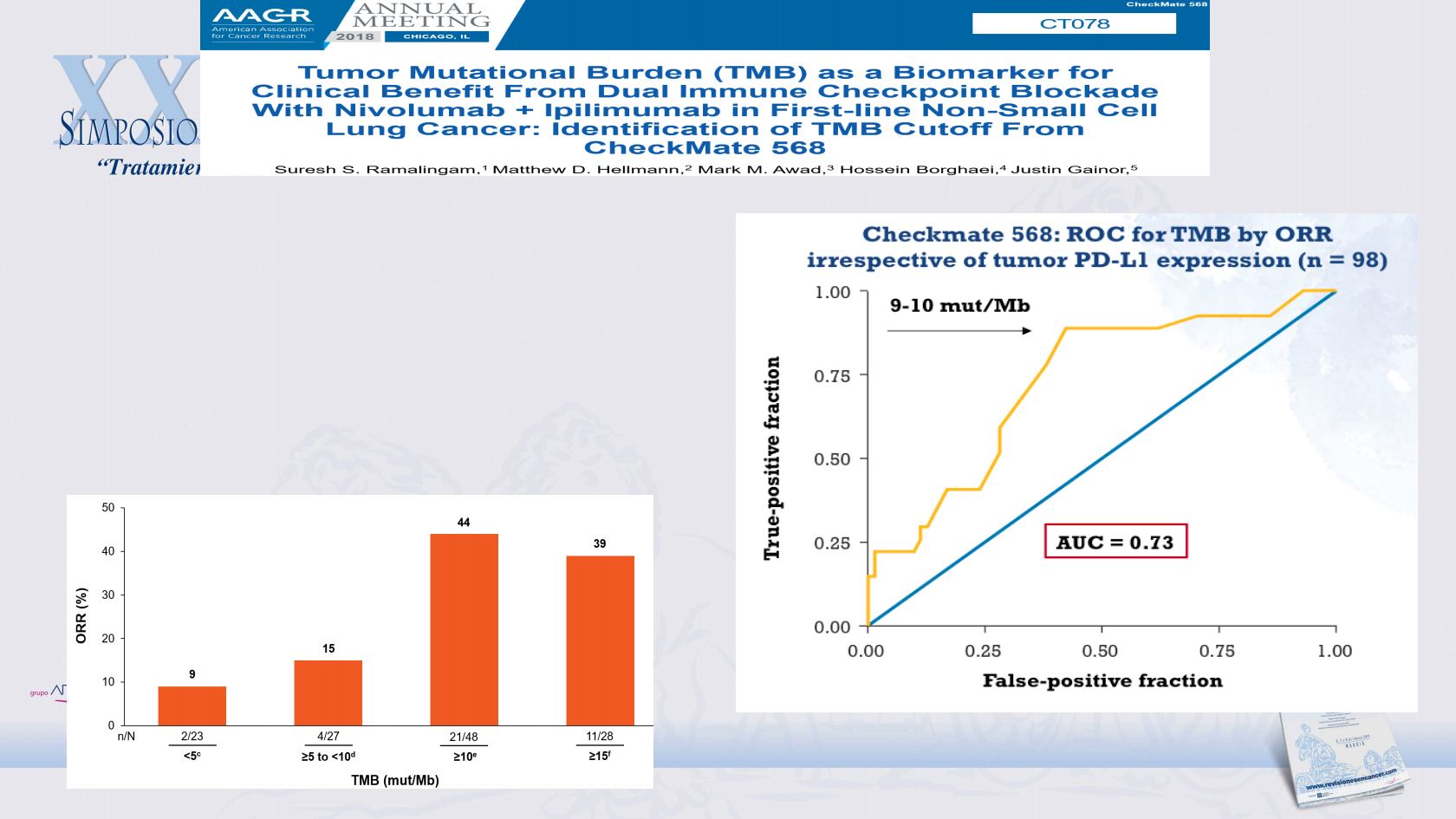
•
ORR increased in patients with higher TMB,
and plateaued at TMB ≥10 mut/Mb
1
1. Ramalingam S et al. Oral presentation at AACR 2018. CT078. 2. Carbone DP et al.
N Engl J Med
. 2017:376;2415–2426. 3. Hellmann MD et al.
Cancer Cell








