 www.revisionesencancer.com
www.revisionesencancer.com
1 )
“Tratamiento médico del cáncer en el año 2019”
Organiza:
6 , 7 y 8 d e f e b r e r o 2 0 1 9
NH Collection Eurobuilding
M A D R I D
www.revisionesencancer.comC O O R D I N A D O R E S C I E N T Í F I C O S :
Eduardo Díaz-Rubio
Enrique Aranda Aguilar
Enrique Grande Pulido
Ana Lluch Hernández
Pedro Pérez Segura
Mariano Provencio Pulla
¿Son todos los test genómicos iguales?
Comparison of the Performance of 6 Prognostic Signatures
for Estrogen Receptor–Positive Breast Cancer
A Secondary Analysis of a Randomized Clinical Trial
IvanaSestak,PhD;RichardBuus,PhD;JackCuzick,PhD;PeterDubsky,MD;RalfKronenwett,MD;
CarstenDenkert,MD;SeanFerree,PhD;DennisSgroi,MD;CatherineSchnabel,PhD;
FrederickL.Baehner,MD;ElizabethMallon,PhD;MitchDowsett,PhD
IMPORTANCE
Multiple molecular signatures are available for managing estrogen receptor
(ER)–positive breast cancer but with little direct comparative information to guide the
patient’s choice.
OBJECTIVE
To conduct a within-patient comparison of the prognostic value of 6 multigene
signatures in women with early ER-positive breast cancer who received endocrine therapy for
5 years.
DESIGN, SETTING, AND PARTICIPANTS
This retrospective biomarker analysis included 774
postmenopausal women with ER-positive
ERBB2
(formerly
HER2
)–negative breast cancer.
This analysis was performed as a preplanned secondary study of data from the Anastrozole or
Tamoxifen Alone or Combined randomized clinical trial comparing 5-year treatment with
anastrozole vs tamoxifen with 10-year follow-up data. The signatures included the Oncotype
Dx recurrence score, PAM50-based Prosigna risk of recurrence (ROR), Breast Cancer Index
(BCI), EndoPredict (EPclin), Clinical Treatment Score, and 4-marker immunohistochemical
score. Data were collected from January 2009, through April 2015.
MAIN OUTCOMES AND MEASURES
The primary objective was to compare the prognostic value
of these signatures in addition to the Clinical Treatment Score (nodal status, tumor size,
grade, age, and endocrine treatment) for distant recurrence for 0 to 10 years and 5 to 10
years after diagnosis. Likelihood ratio (LR) statistics were used with the χ
2
test and C indexes
to assess the prognostic value of each signature.
RESULTS
In this study of 774 postmenopausal women with ER-positive,
ERBB2
-negative
disease (mean [SD] age, 64.1 [8.1] years), 591 (mean [SD] age, 63.4 [7.9] years) had
node-negative disease. The signatures providing the most prognostic information were the
ROR (hazard ratio [HR], 2.56; 95% CI, 1.96-3.35), followed by the BCI (HR, 2.46; 95% CI,
1.88-3.23) and EPclin (HR, 2.14; 95% CI, 1.71-2.68). Each provided significantly more
information than the Clinical Treatment Score (HR, 1.99; 95% CI, 1.58-2.50), the recurrence
score (HR, 1.69; 95% CI, 1.40-2.03), and the 4-marker immunohistochemical score (HR, 1.95;
95% CI, 1.55-2.45). Substantially less information was provided by all 6 molecular tests for the
183 patients with 1 to 3 positive nodes, but the BCI (ΔLR χ
2
= 9.2) and EPclin (ΔLR χ
2
= 7.4)
provided more additional prognostic information than the other signatures.
CONCLUSIONS AND RELEVANCE
For women with node-negative disease, the ROR, BCI, and
EPclin were significantly more prognostic for overall and late distant recurrence. For women
with 1 to 3 positive nodes, limited independent information was available from any test.
These data might help oncologists and patients to choose the most appropriate test when
considering chemotherapy use and/or extended endocrine therapy.
TRIAL REGISTRATION
isrctn.comIdentifier:
ISRCTN18233230
Supplementalcontent
AuthorAffiliations:
Author
affiliationsarelistedattheendofthis
article.
CorrespondingAuthor:
Ivana
Research
JAMA Oncology |
Original Investigation
and includes information on tumor size. The TransATAC co- groups. To compare the prognostic
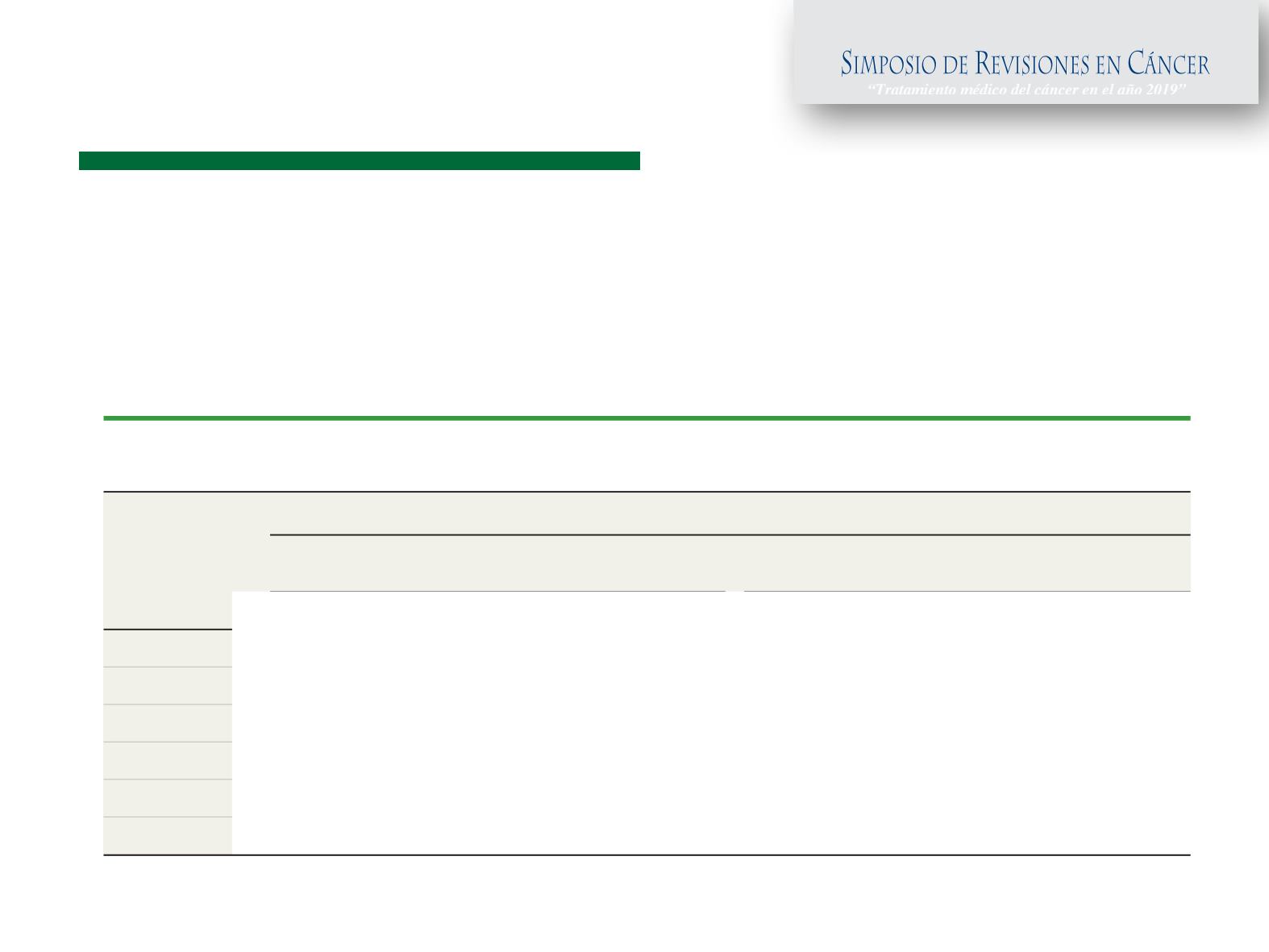
Table 1. Univariate HRs and C Indexes for All Prognostic Signatures According to Nodal Status
During Years 0 to 10
Gene
Signature
Patient Group
No e-Negative Disease
(n = 591)
Node-Positive Disease
(n = 227)
HR (95% CI)
a
C Index (95% CI)
HR (95% CI)
a
C Index (95% CI)
CTS
1.99 (1.58-2.50)
0.721 (0.668-0.774)
1.63 (1.20-2.21)
0.640 (0.554-0.726)
IHC4
1.95 (1.55-2.45)
0.725 (0.665-0.785)
1.33 (0.99-1.78)
0.601 (0.511-0.690)
RS
1.69 (1.40-2.03)
0.667 (0.585-0.750)
1.39 (1.05-1.85)
0.603 (0.513-0.693)
BCI
2.46 (1.88-3.23)
0.762 (0.704-0.820)
1.67 (1.21-2.29)
0.652 (0.566-0.739)
ROR
2.56 (1.96-3.35)
0.764 (0.707-0.821)
1.58 (1.16-2.15)
0.636 (0.552-0.719)
EPclin
2.14 (1.71-2.68)
0.765 (0.716-0.814)
1.69 (1.29-2.22)
0.671 (0.590-0.752)
Abb
Inde
EPcl
HR,
imm
ROR
RS, r
a
All
Prognostic Signatures for Estrogen Receptor–Positive Breast Cancer
HRHA
HAZARD RATIO (IC95%): HR
CONCORDANCE INDEX: C-INDEX
≤0.5 MALO O NO PREDICE
>0.7 MODELO ROBUSTO








