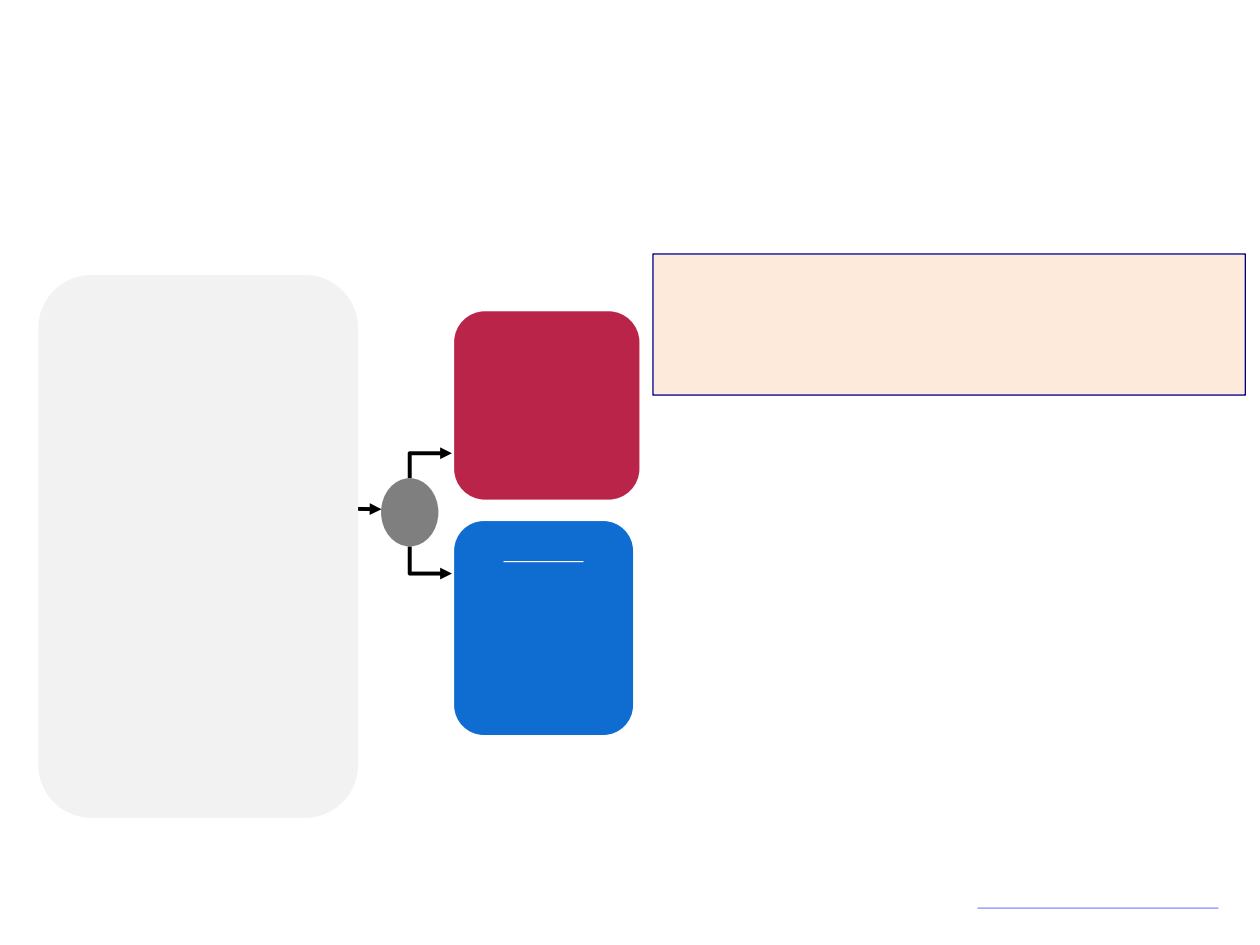
ARCHES
Placebo
With LHRH
agonist/antago
nist or
bilateral
orchiectomy
Enzalutamide
160 mg QD
With LHRH
agonist/antago
nist or
bilateral
orchiectomy
R
1:1
N=1150
•
mPC documented on
bone scan/ CT or MRI
•
Prior treatment with
docetaxel (up to 6 cycles)
•
ADT (LHRH) up to 3
month (or 6 month with
docetaxel) is allowed -
No radiographic evidence
of disease progression or
rising PSA levels prior to
day 1
•
Prior ADT given for < 39
months in duration and >
9 months before
randomization as
neoadjuvant/adjuvant
therapy.
Primary Endpoints
rPFS
(based on central review)
Secondary Endpoints
Overall Survival
Time to first SSE
Time to castration resistance
Time to deterioration of QoL
Time to initiation of new antineoplastic
therapy
Time to PSA progression (≥ 2 ng/mL)
PSA undetectable rate (< 0.2 ng/mL)
ORR
Time to pain progression
Safety Endpoints
AEs, labs, vitals, ECG, physical exams
STRATIFICATION: Volume of disease (high vs. low) and prior docetaxel
therapy (0, 1-5, 6 cycles)
www.clinicaltrials.gov(NCT02677896).
Phase III, Randomized, Double-blind study in mHSPC








