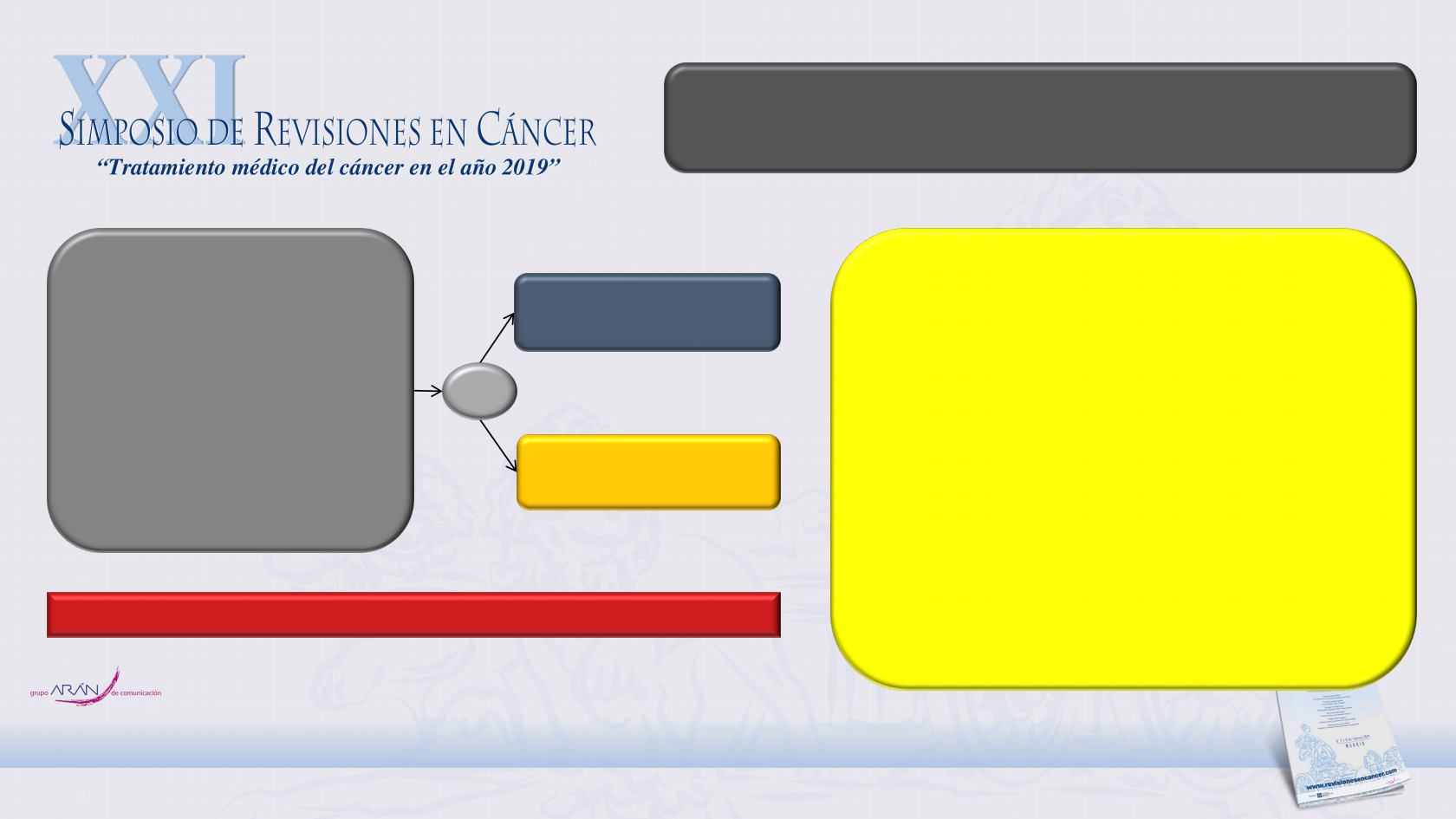
ClinicalTrials.gov: NCT02003924
Enzalutamide
160 mg QD
Placebo
QD
R
2:1
N=1401
Non-metastatic (M0) CRPC
Testosterone
≤50 ng/dL
Progressive disease with
ongoing ADT
Asymptomatic
PSADT ≤10 months
•
PROSPER is a multinational, phase 3, randomised, double-blind,
placebo-controlled trial
•
Primary endpoint: Metastasis-free survival
Primary endpoint: METASTASIS-FREE SURVIVAL BENEFIT
Secondary endpoints:
•
OS
•
Time to pain progression
•
Time to opiate use for prostate
cancer pain
•
Time to first use of cytotoxic chemotherapy
•
Time to first use of new
antineoplastic therapy
•
Time to PSA progression
•
PSA response rates
•
QoL
•
Safety
Hussain M, et al. N Engl J Med 2018;378:2465-74








