Efficacy of Tivozanib after sorafenib:
crossover of a phase 3 study
Molina A, et al. Eur J Cancer 2018
the lack of available second-line therapies at the time,
many patients in the TIVO-1 study randomised to
tivozanib in the Eastern European countries had no
salvage therapy. In the current analysis, anti-tumour
activity of tivozanib in the 161 patients who crossed over
after radiographic progression on sorafenib was
demonstrated with a median PFS of 11 months (95% CI:
7.3
e
12.7 months), a median overall survival of
(26%) than for patients in TIVO-1 treated with tivozanib
(44%
[17]
). The lower incidence of hypertension in this
study may be secondary to a selection bias, or perhaps
to closer monitoring and management of this class ef-
fect, particularly as patients came off sorafenib. In
addition, more than half of the patients enrolled in this
study completed treatment without dose reductions or
interruptions, with a relative dose intensity of 95%.
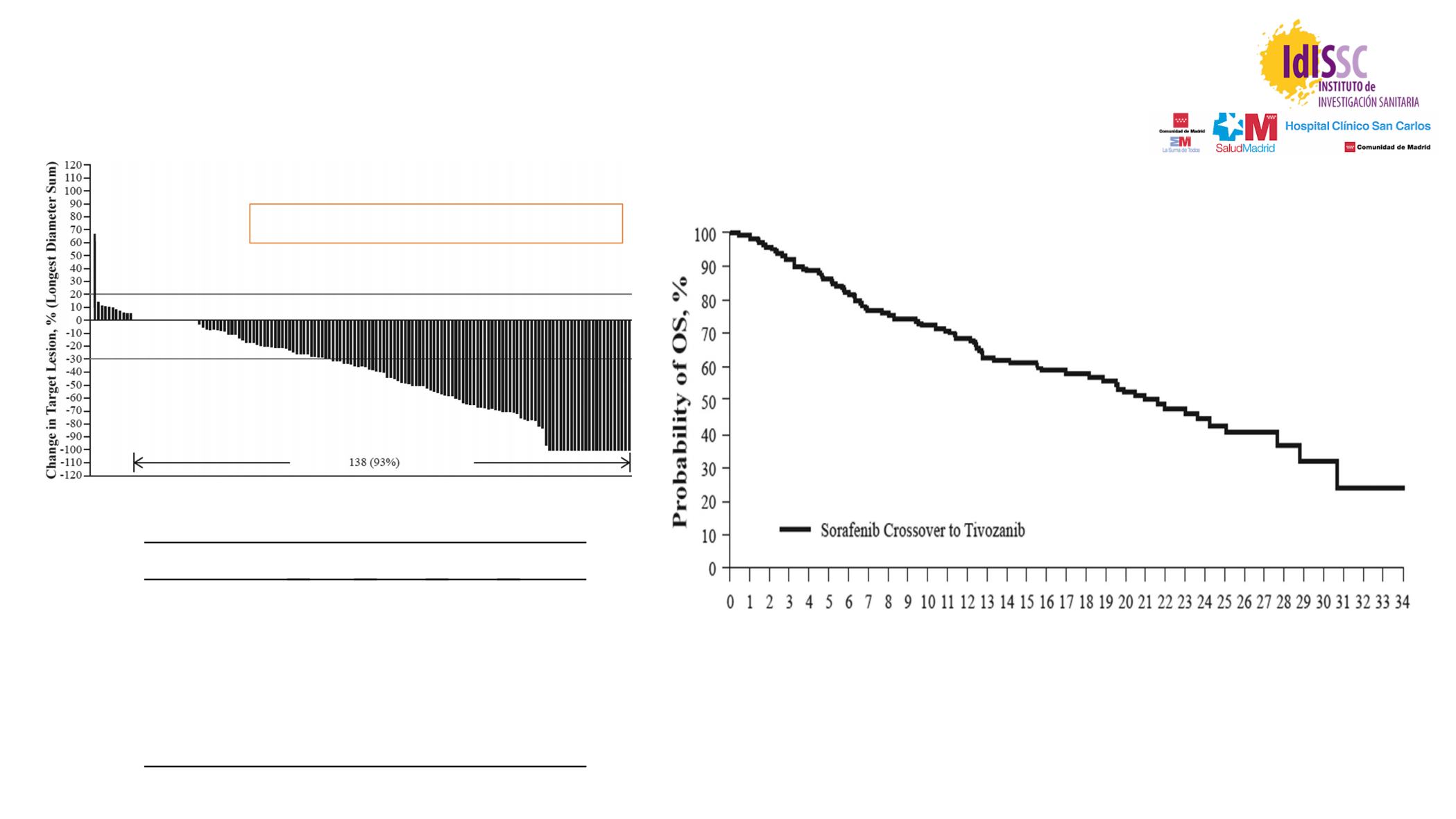
Fig. 2. Maximum percentage change in target lesion diameter from baseline of crossover study; evaluable population (N
Z
149).
The most common treatment-related AEs observed in was assessed by the investigator as possibly related to
Fig. 1. Kaplan
e
Meier curves of investigator-associated disease progression (A) and overall survival (B). PFS, progression-free survival;
OS, overall survival.
profiles were found to be acceptable and consistent with
the established AE profile of tivozanib.
The nature of the study design introduced the study
limitations common to all single-arm studies, namely the
fact that there was no control group for comparison. In
addition, a specific limitation is that the study included
only patients with Eastern Cooperative Oncology
Group performance status of 0 or 1 and therefore, did
not include poor prognosis patients. This may limit the
ability to extend these results to the general patient
population.
5. Conclusions
In this study reporting crossover data from a rando-
mised, phase 3 clinical trial inv stigating tivozanib and
Table 4
Most frequently reported AEs occurring in
>
5% of patients by
preferred term in crossover evaluable population (N
Z
161).
a
AE, n (%)
Grade
1
e
2
Grade
3
Grade
4
Total
Hypertension
23 (14) 18 (11)
0 (0)
41 (26)
Diarrhoea
18 (11) 4 (2)
0 (0)
22 (13)
Fatigue
14 (9)
7 (4)
0 (0)
21 (13)
Asthenia
14 (9)
6 (4)
0 (0)
20 (12)
Palmar-plantar
erythrodysesthesia
syndrome
14 (9)
2 (1)
0 (0)
16 (10)
Cough
9 (6)
0 (0)
0 (0)
9 (6)
Dysphonia
9 (6)
0 (0)
0 (0)
9 (6)
Decreased appetite
9 (6)
0 (0)
0 (0)
9 (6)
Dyspnoea
7 (4)
2 (1)
0 (0)
9 (6)
AE, adverse event; MedDRA, Medical Dictionary for Regulatory
Activities.
A.M. Molina et al. / European Journal of Cancer 94 (2018) 87
e
94
93
from the start of the first tivozanib dose in this study
was 22 months (95% CI: 17.0
e
27.6 months;
Fig. 1
B).
None of the crossover patients had a complete response;
29 (18%) had a partial response, 83 (52%) had stable
disease and 34 (21%) had progressive disease (
Table 3
).
The median duration f response for the 29 responders
was 15 m nths (95% CI:
!
11.1 months). Among these
evaluable p tients, the confirmed overall response rate
was 18% (95% CI: 12.4
e
28.8). There were an additional
15 (9%) unevaluable patients. A total of 149 of 161
crossover patients had measurable disease post baseline.
Of these patients, 138 (92.6%) had a reduction in target
lesion diameter (
Fig. 2
).
A total of 124 of 161 (77%) crossover patients expe-
rienced AEs; 86 (53%) had treatment-related AEs, and
12 (13%) had AEs leading to death. grade
!
3 AEs were
reported by 48% of patients, including 24% that were
treatment related. A total of 30% of patients had serious
AEs, 4% of which were treatment related. About 4% of
patients discontinued treatment because of AEs.
Evaluable population
(N
Z
161)
46 (20)
115 (71)
59.0
23
e
85
127 (79)
34 (21)
156 (97)
5 (3)
92 (57)
69 (43)
143 (89)
Europe
10 (6)
8 (5)
rative Oncology Group performance status.
erwise specified.
after the first documented progressive disease and 14
patients who continued on sorafenib on entering this
study and subsequently progressed and crossed over to
tivozanib. Baseline characteristics were generally
balanced between the study groups (
Table 1
). The ma-
jority of patients were male and white, had a median age
of 59 years (range: 23
e
86) and were fromCentral/Eastern
Europe, as shown in the baseline characteristics. At the
time of analysis, 125 (78%) patients discontinued the
study and 36 (22%) patients were ongoing (
Table 2
). The
most common reason for treatment discontinuation was
progressive disease (56%). The median number of cycles
in crossover patients exposed to tivozanib was eight cycles
(range: 1
e
35) over a median of 225 days (range: 15
e
172).
The mean total dose administered was 318.84 mg, and the
relative dose intensity was 95%, with over half of the
patients receiving the full dose of tivozanib throughout
the course of therapy. Treatment interruptions due to
AEs were observed in 25 (16%) patients and dose re-
ductions due to AEs in 15 (9%) patients.
Anti-tumour activity with tivozanib was demon-
strated with a median PFS of 11 months (95% CI:
7.3
e
12.7 months;
Fig. 1
A). Median overall survival
from the start of the first tivozanib dose in this study
was 22 months (95% CI: 17.0
e
27.6 months;
Fig. 1
B).
None of the crossover patients had a complete response;
29 (18%) had a partial response, 83 (52%) had stable
disease and 34 (21%) had progressive disease (
Table 3
).
The median duration of response for the 29 responders
was 15 months (95% CI:
!
11.1 months). Among these
evaluable patients, the confirmed overall response rate
was 18% (95% CI: 12.4
e
28.8). There were an additional
15 (9%) unevaluable patients. A total of 149 of 161
crossover patients had measurable disease post baseline.
Of these patients, 138 (92.6%) had a reduction in target
lesion diameter (
Fig. 2
).
A total of 124 of 161 (77%) crossover patients expe-
rienced AEs; 86 (53%) had treatment-related AEs, and
12 (13%) had AEs leading to death. grade
!
3 AEs were
reported by 48% of patients, including 24% that were
treatment related. A total of 30% of patients had serious
AEs, 4% of which were treatment related. About 4% of
patients discontinued treatment because of AEs.
Table 1
Baseline characteristics of crossover patients from study TIVO-1 to
study 902 (N
Z
161).
Baseline characteristics
Evaluable population
(N
Z
161)
Gender
Female
46 (20)
Male
115 (71)
Age
Median
59.0
Range
23
e
85
Age group
<
65 years
127 (79)
!
65 years
34 (21)
Race
White
156 (97)
Asian
5 (3)
ECOG PS
0
92 (57)
1
69 (43)
Geographic region
Central/Eastern Europe
143 (89)
North America/Western Europe
10 (6)
Rest of world
8 (5)
ECOG PS, Eastern Cooperative Oncology Group performance status.
Data are n (%), unless otherwise specified.
a
Reasons for discontinuation were based on the end-of-treatment
electronic case report form page.
b
Eight patients had death as the reason for discontinuation.
c
Four patients discontinued but agreed to continue on study in the
follow-up, and one patient was withdrawn because of an investigator’s
mistake in calculation of the sum of the longest diameters.
after the first documented progressive disease and 14
patients who continued on sorafenib on entering this
study and subsequently progr ssed and crossed over to
tivozanib. Baseline char cteristi s w re gen rally
balanced between the study groups (
Table 1
). The ma-
jority of patients were male and white, had a median age
of 59 years (range: 23
e
86) and were fromCentral/Eastern
Europe, as shown in the baseline characteristics. At the
time of analysis, 125 (78%) patients disco tinue the
study and 36 (22%) patients were ongoing (
T ble 2
). The
most common reason for treatment discontinuation was
progressive disease (56%). The median number of cycles
in crossover patients exposed to tivozanib was eight cycles
(range: 1
e
35) over a me
The mean total dose ad
relative dose intensity
patients receiving the f
the course of therapy.
AEs were observed in
ductions due to AEs in
Anti-tumour activit
strated with a median
7.3
e
12.7 months;
Fig.
from the start of the fi
was 22 months (95% C
None of the crossover p
29 (18%) had a partial
disease and 34 (21%) h
The median duration of
was 15 months (95% C
evaluable patients, the
was 18% (95% CI: 12.4
15 (9%) unevaluable p
crossover patients had
Of these patients, 138 (
lesi
diameter (
Fig. 2
).
A total of 124 of 16
rienced AEs; 86 (53%)
12 (13%) had AEs leadi
reported by 48% of pa
treatment related. A tot
AEs, 4% of which were
patients discontinued tr
Table 1
Baseline characteristics of crossover patients from study TIVO-1 to
study 902 (N
Z
161).
Baseline characteristics
Evaluable population
(N
Z
161)
Gender
Female
46 (20)
Male
115 (71)
Age
Median
59.0
Range
23
e
85
Age group
<
65 years
127 (79)
!
65 years
34 (21)
Race
White
156 (97)
Asian
5 (3)
ECOG PS
0
92 (57)
1
69 (43)
Geographic region
Central/Eastern Europe
143 (89)
North America/Western Europe
10 (6)
Res of world
8 (5)
ECOG PS, Eastern Cooperative Oncology Group performance status.
Data are n (%), unless otherwise specified.
electronic case report form pa
b
Eight patients had death a
c
Four patients discontinue
foll w-up, and on patient wa
mistake in calculation of the








