Motzer R, et al. J Clin Oncol 2013
Tivozanib vs Sorafenib: Phase III trial
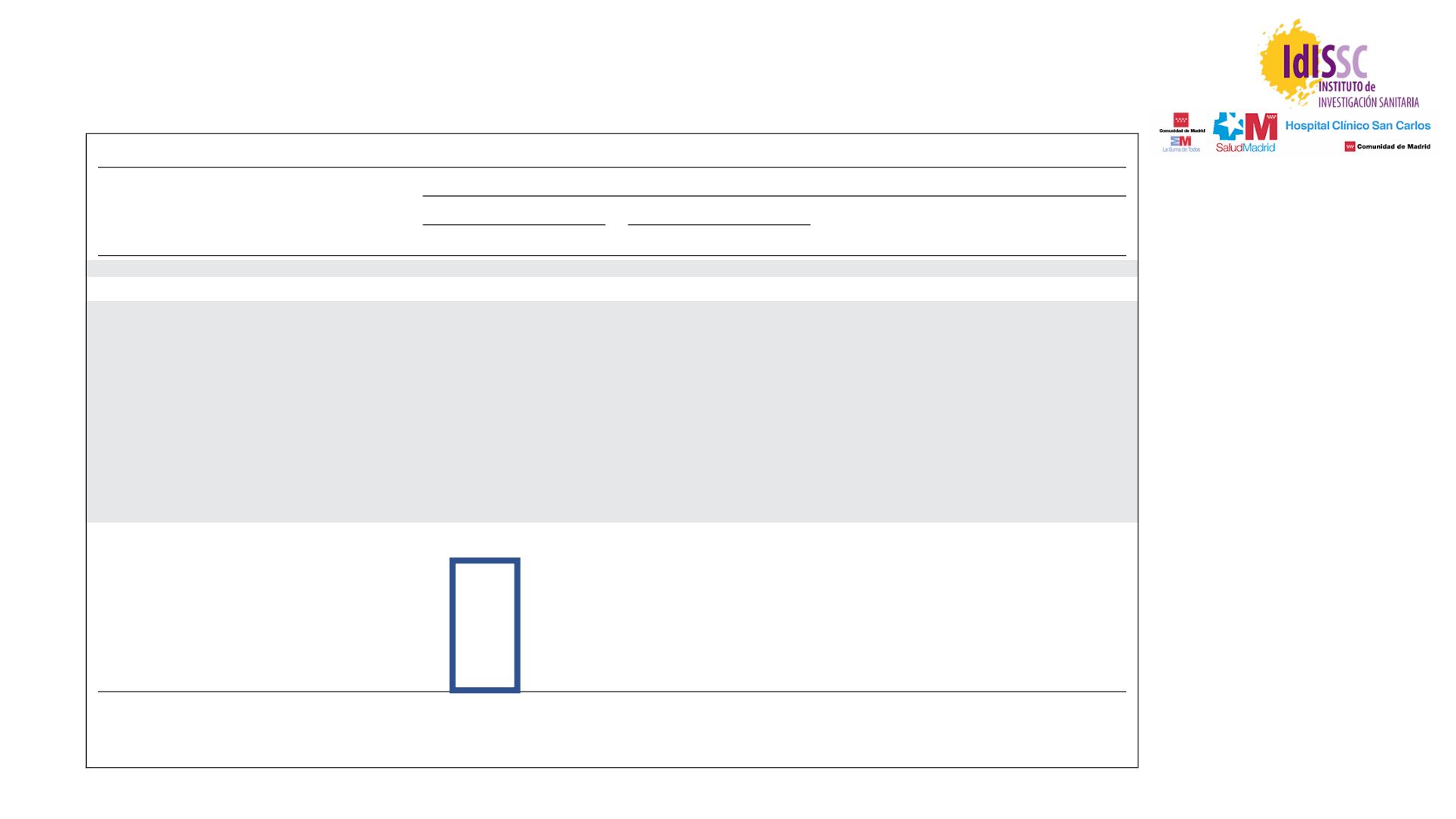
Table 2.
Summary of Efficacy Measures in Intent-to-Treat Population
Efficacy Measure
Independent Radiology Review
Tivozanib (n
!
260)
Sorafenib (n
!
257)
HR for Progression
or Death
95% CI
P
No.
% 95% CI
No.
% 95% CI
PFS
Overall estimated median PFS, months
11.9
9.3 to 14.7 9.1
7.3 to 9.5
0.797
0.639 to 0.993 .042
Stratified estimated median PFS, months
Prior treatment
No prior treatment
12.7
9.1 to 15.0 9.1
7.3 to 10.8
0.756
0.580 to 0.985 .037
Prior systemic therapy for metastatic RCC 11.9
8.0 to 16.6 9.1
7.2 to 11.1
0.877
0.587 to 1.309 .520
ECOG PS
0
14.8
11.3 to N/A 9.1
7.5 to 11.0
0.617
0.442 to 0.860 .004
1
9.1
7.5 to 12.9 9.0
7.2 to 10.9
0.920
0.680 to 1.245 .588
MSKCC prognostic group
Favorable
16.7
14.7 to N/A 10.8
9.0 to 16.5
0.590
0.378 to 0.921 .018
Intermediate
9.4
8.2 to 13.0 7.4
7.1 to 9.2
0.786
0.601 to 1.028 .076
Poor
!
3.7
1.9 to 7.4 10.9
5.3 to 11.0
1.361
0.546 to 3.393 .504
Tumor response
Best observed RECIST response
Complete response
3
1.2
2
0.8
—
—
Partial response
83 31.9
58 22.6
—
—
Stable disease
134 51.5
168 65.4
—
—
Progressive disease
34 13.1
19
7.4
—
—
Not evaluable
6
2.3
10
3.9
—
—
Objective response rate
86 33.1 27.4 to 39.2 60 23.3 18.3 to 29.0
—
.014
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; MSKCC, Memorial Sloan-Kettering Cancer Center; N/A, not
achieved; PFS, progression-free survival; RCC, renal cell carcinoma.
!
Based on 17 patients given tivozanib and 10 patients given sorafenib, corresponding to 5% of patients enrolled onto the trial.
Motzer et al








