Entrectinib: safety overview
Overall safety population (N=355)
– Most adverse events were Grade 1/2
and reversible
– Treatment-related AEs leading to
•
dose reduction: 27.3%
•
dose interruption: 25.4%
•
discontinuation
from treatment:
3.9%
– No grade 5 treatment-related events
Treatment-related AEs in the
NTRK
fusion-positive safety
population are consistent with
the overall safety population
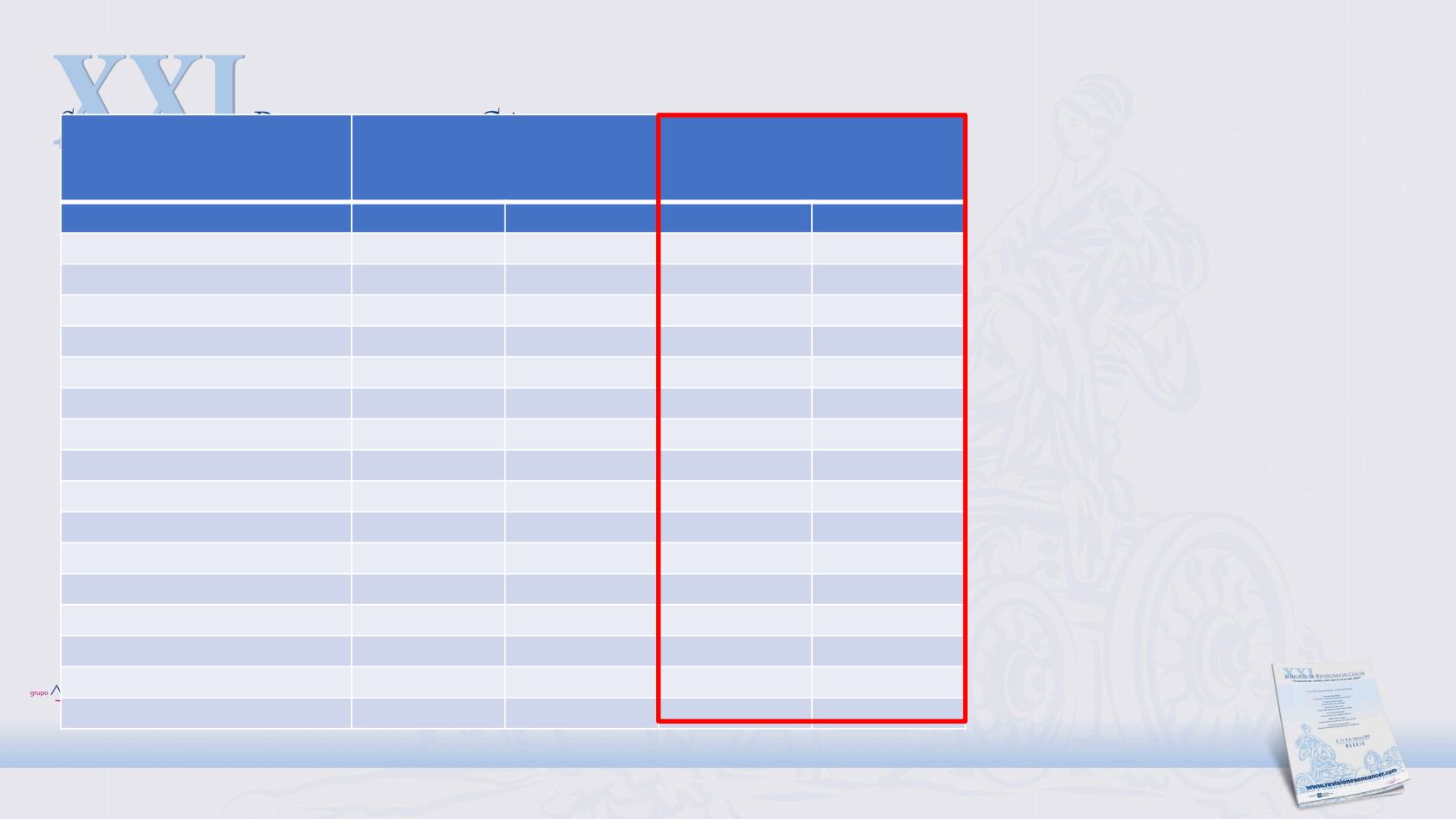
Treatment-related AEs
reported in ≥10% of
patients
NTRK
fusion-positive safety
population (n=68)*
Overall safety population
(N=355)
Patients, n (%)
Grade 1/2
Grade 3
Grade 1/2
Grade 3
Dysgeusia
32 (47.1)
0
146 (41.1)
1 (0.3)
Constipation
19 (27.9)
0
83 (23.4)
1 (0.3)
Fatigue
19 (27.9)
5 (7.4)
89 (25.1)
10 (2.8)
Diarrhoea
18 (26.5)
1 (1.5)
76 (21.4)
5 (1.4)
Oedema peripheral
16 (23.5)
1 (1.5)
49 (13.8)
1 (0.3)
Dizziness
16 (23.5)
1 (1.5)
88 (24.8)
2 (0.6)
Blood creatinine increase
12 (17.6)
1 (1.5)
52 (14.6)
2 (0.6)
Paraesthesia
11 (16.2)
0
67 (18.9)
0
Nausea
10 (14.7)
0
74 (20.8)
0
Vomiting
9 (13.2)
0
48 (13.5)
0
Arthralgia
8 (11.8)
0
42 (11.8)
2 (0.6)
Myalgia
8 (11.8)
0
52 (14.6)
2 (0.6)
Weight increased
8 (11.8)
7 (10.3)
51 (14.4)
18 (5.1)
AST increase
§
7 (10.3)
0
35 (9.9)
3 (0.8)
Muscular Weakness
6 (8.8)
1 (1.5)
22 (6.2)
3 (0.8)
Anaemia
5 (7.4)
8 (11.8)
27 (7.6)
16 (4.5)
Cut-off date: 31 May 2018
*
NTRK
fusion-positive safety population comprises all patients who have received at least 1 dose of entrectinib regardless of dose or follow up
§
One Grade 4 treatment-related AE (AST increase)








