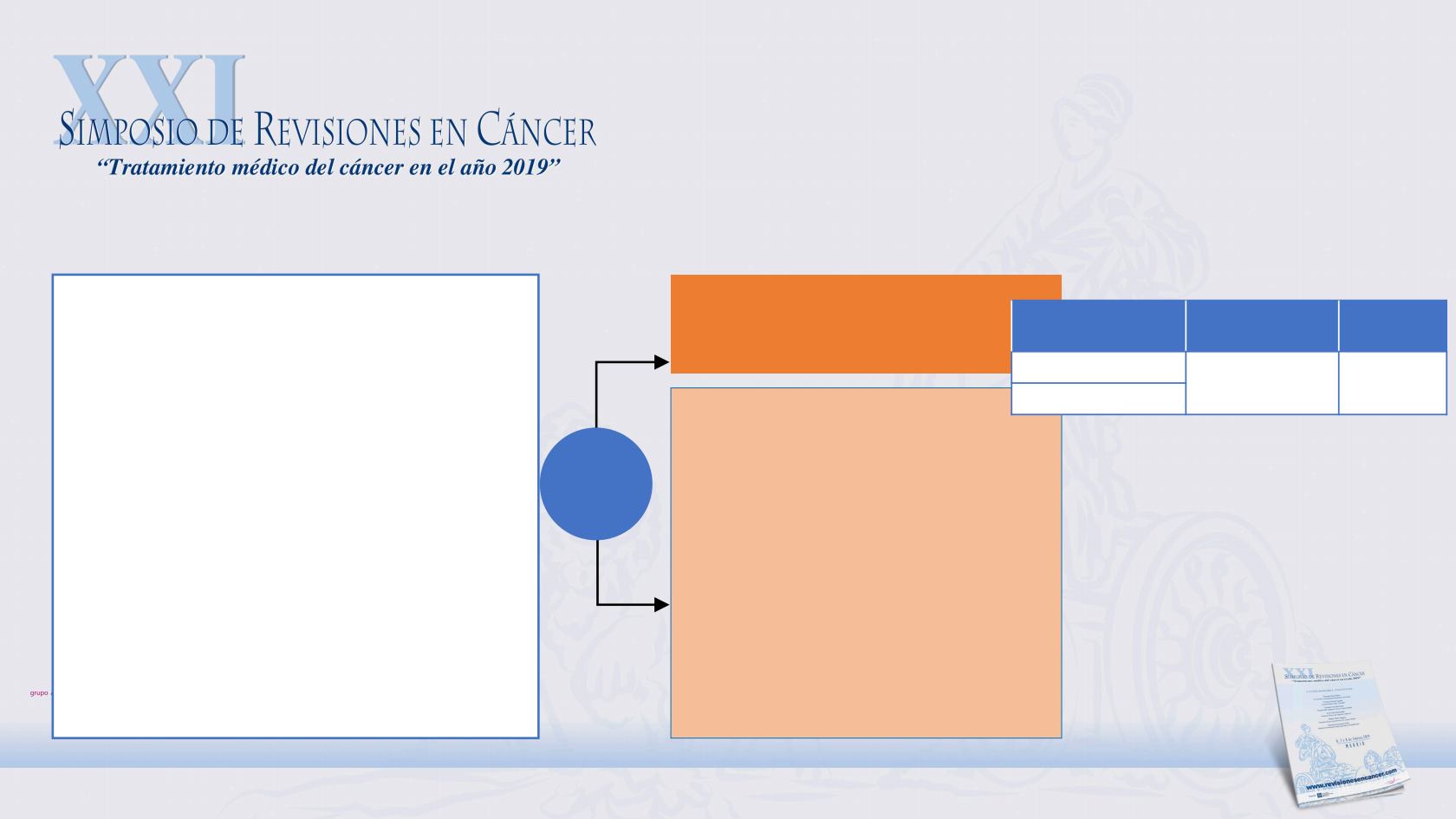
CheckMate 141
R
2:1
Nivolumab
3 mg/kg IV Q2W
Investigator’s Choice
• Methotrexate 40 mg/m² IV
weekly
• Docetaxel 30 mg/m² IV weekly
• Cetuximab 400 mg/m² IV
once, then 250 mg/m² weekly
Key eligibility criteria
• R/M HNSCC of the oral cavity, pharynx,
or larynx
• Progression on or within 6 months of last
dose of platinum-based therapy
• Irrespective of no. of prior lines of
therapy
• Documentation of p16 to determine HPV
status (oropharyngeal)
• Regardless of PD-L1 status
a
Stratification factor
• Prior cetuximab treatment
Randomised, global, Phase III trial of efficacy and
safety of nivolumab versus investigator’s choice
Ferris RL, et al.
N Engl J Med
2016;375:1856–1867.
Median OS, mo (95%
CI)
HR
(97.73% CI)
p-value
7.5 (5.5, 9.1)
0.70
(0.51, 0.96)
0.0101
5.1 (4.0, 6.0)








